Abstract
Short-term plasticity is a fundamental synaptic property thought to underlie memory and neural processing. The glomerular microcircuit comprises complex excitatory and inhibitory interactions and transmits olfactory nerve signals to the excitatory output neurons, mitral/tufted cells (M/TCs). The major glomerular inhibitory interneurons, short axon cells (SACs) and periglomerular cells (PGCs), both provide feedforward and feedback inhibition to M/TCs and have reciprocal inhibitory synapses between each other. Olfactory input is episodically driven by sniffing. We hypothesized that frequency-dependent short-term plasticity within these inhibitory circuits could influence signals sent to higher-order olfactory networks. To assess short-term plasticity in glomerular circuits and MC outputs, we virally delivered channelrhodopsin-2 (ChR2) in glutamic acid decarboxylase-65 promotor (GAD2-cre) or tyrosine hydroxylase promoter (TH-cre) mice and selectively activated one of these two populations while recording from cells of the other population or from MCs. Selective activation of TH-ChR2-expressing SACs inhibited all recorded GAD2-green fluorescent protein(GFP)-expressing presumptive PGC cells, and activation of GAD2-ChR2 cells inhibited TH-GFP-expressing SACs, indicating reciprocal inhibitory connections. SAC synaptic inhibition of GAD2-expressing cells was significantly facilitated at 5–10 Hz activation frequencies. In contrast, GAD2-ChR2 cell inhibition of TH-expressing cells was activation-frequency independent. Both SAC and PGC inhibition of MCs also exhibited short-term plasticity, pronounced in the 5–20 Hz range corresponding to investigative sniffing frequency ranges. In paired SAC and olfactory nerve electrical stimulations, the SAC to MC synapse was able to markedly suppress MC spiking. These data suggest that short-term plasticity across investigative sniffing ranges may differentially regulate intra- and interglomerular inhibitory circuits to dynamically shape glomerular output signals to downstream targets.
NEW & NOTEWORTHY Short-term plasticity is a fundamental synaptic property that modulates synaptic strength based on preceding activity of the synapse. In rodent olfaction, sensory input arrives episodically driven by sniffing rates ranging from quiescent respiration (1–2 Hz) through to investigative sniffing (5–10 Hz). Here we show that glomerular inhibitory networks are exquisitely sensitive to input frequencies and exhibit plasticity proportional to investigative sniffing frequencies. This indicates that olfactory glomerular circuits are dynamically modulated by episodic sniffing input.
Keywords: mitral cells, network, olfactory bulb, periglomerular cells, short axon cells, STP
INTRODUCTION
Short-term plasticity is a fundamental synaptic property thought to underlie memory and neural processing (Ryan et al. 2015). Unlike most brain areas, inhibitory interneurons significantly outnumber output neurons in the olfactory bulb. Thus, short-term plasticity in glomerular inhibitory circuits could play a major role in olfactory signal processing. Glomerular microcircuits integrate complex excitatory and inhibitory interactions and transmit olfactory nerve signals to the excitatory output neurons, mitral/tufted cells (M/TCs), at the initial stage of olfactory processing (Harvey and Heinbockel 2018). The major glomerular inhibitory interneuron types are short axon cells (SACs) and periglomerular cells (PGCs). Both provide feedforward and feedback inhibition to M/TCs and have reciprocal inhibitory synapses between each other (Shao et al. 2019). Because olfactory input is episodically driven by sniffing, we hypothesized that frequency-dependent short-term plasticity within glomerular inhibitory circuits could significantly influence output signals sent to higher-order olfactory networks.
Operations of glomerular circuitry are complex. Olfactory nerve (ON) inputs monosynaptically excite external tufted cells (ETCs) as well as M/TCs, comprising a direct glomerular throughput circuit (De Saint Jan et al. 2009; Gire et al. 2012; Gire and Schoppa 2009; Hayar et al. 2004a, 2004b, 2005; Najac et al. 2011). Additionally, ON inputs directly (and indirectly) excite PGCs and SACs to provide feedforward inhibition of ETCs and M/TCs (Liu et al. 2016). PGCs form GABAergic inhibitory circuits within a single glomerulus (Price and Powell 1970; Shao et al. 2012, 2013). In contrast, SACs release GABA and dopamine (DA) and contact multiple glomeruli to form an interglomerular circuit. Activation of this circuit inhibits neurons in multiple glomeruli (Aungst et al. 2003; Banerjee et al. 2015; Borisovska et al. 2013; Kiyokage et al. 2010; Kosaka and Kosaka 2008; Liu et al. 2013, 2016; Whitesell et al. 2013). Together, intra- and interglomerular inhibition modulate the duration and spike frequency of output neurons (Shao et al. 2012).
In addition to their static complexity, glomerular circuits are also dynamically regulated. Inhaled odorants are episodic, varying with an animal’s inhalation cycle, i.e., sniffing, which in rodents ranges in frequency from 1–2 Hz in basal respiration to 5–10 Hz during investigatory sniffing (Carey and Wachowiak 2011; Verhagen et al. 2007; Wachowiak 2011; Wesson et al. 2008). Sniff sampling occurs in the same frequency range as short-term synaptic plasticity and may engage functionally relevant short-term plasticity of glomerular inhibitory circuits. However, very little is known about short-term plasticity of SAC and PGC synapses, despite the observation that glomeruli contain large numbers of inhibitory interneurons (Parrish-Aungst et al. 2007).
We crossed Cre and GFP transgenic mice combined with virally delivered optogenetic constructs to express light-gated cation channel channelrhodopsin-2 (ChR2) in glutamic acid decarboxylase (GAD) and tyrosine hydroxylase (TH)-cre mice. TH expression is a reliable marker for SACs. GAD2 is expressed by the vast majority of PGCs, but a small subset of SACs express GAD2 in addition to TH. Thus, activation of GAD2 cells may engage some SACs. However, as the present results show marked differences between TH- and GAD2-cell synapses, the potential confound of GAD2 activation of SACs appears to be limited. We found that TH- and GAD2-expressing neurons mutually inhibit each other, and activation of either cell type evoked short, invariant latency inhibition of mitral cells (MCs). Additionally, short-term facilitation in both intra- and interglomerular circuits markedly suppresses MC spiking. This suggests that glomerular inhibitory synapses are facilitated by inputs at sniffing frequency ranges. Thus, sniffing may engage short-term plasticity to dynamically shape olfactory bulb output signals.
MATERIALS AND METHODS
Animals.
Mice used in this study include a transgenic-expressing green fluorescent protein (GFP) under the control of the glutamic acid decarboxylase-65 promotor (GAD65-GFP; courtesy of Dr. Gabor Szabo, Hungary) or under the control of the tyrosine hydroxylase promoter (TH-GFP; courtesy of Dr. Kazuto Kobayashi, Japan) and transgenic mice expressing cre recombinase under the glutamic acid decarboxylase-65 promotor (GAD2-cre; Jax mice strain B6.129.GAD2-cre) or under the control of the tyrosine hydroxylase promoter [TH-cre; Jax mice strain B6.Cg-Tg(Th-cre)1Tmd/J]. Double heterozygote mice were generated by crossing a heterozygote TH-GFP with a heterozygote GAD2-cre or a heterozygote GAD65-GFP with a heterozygote TH-cre. Colonies of transgenic mice were maintained by breeding wild-type C57BL/6J female mice with a heterozygous male TH-GFP, TH-cre, or GAD2-cre or a wild-type B6CBAF1/J with a heterozygous male GAD65-GFP. Approximately equal numbers of male and female mice were utilized in the experiments. Analysis of the responses in each experiment did not show evidence of sex differences; thus, results from male and female animals were pooled in the reported sample number n for each experiment. All animal colonies and experimental procedures were performed in accordance with protocols approved by the University of Maryland Institutional Animal Care and Use Committee.
Channelrhodopsin-2 expression.
The optogenetic construct channelrhodopsin-2 (ChR2) was expressed in GAD2-cre/TH-GFP or TH-cre/GAD65-GFP as described previously (Liu et al. 2016). Briefly, the Cre-inducible adeno-associated virus serotype 2.9 (AAV2.9) carrying a fusion construct of ChR2 and enhanced yellow [AAV2.9-hSyn-hChR2(H134R)-EYFP] or mCherry fluorescent protein [AAV-hSyn-hChR2(H134R)-mCherry, University of Pennsylvania Vector Core] (Tsai et al. 2009) was injected into the glomerular layer of the medial side of each olfactory bulb (OB) at 4–5 postnatal weeks. Once mice were under anesthesia, the skull was exposed and two small holes (0.5 mm diameter) were drilled for injection. The OB coordinates for 6 injection sites were bregma, +4.3; lateral, ± 0.3; and depth, −2.2, −1.6, and −1.0 mm. AAV2.9 injections of 0.25 µL were performed over 5 min per injection site. After at least 3 wk for ChR2 expression, slices were prepared for electrophysiology experiments.
Slice preparation.
Animals were anesthetized with saturated vapor isoflurane and the olfactory bulbs removed. The bulbs were immediately secured to a cutting platform and immersed in 4°C oxygenated cutting solution containing the following (in mM): 26 NaHCO3, 1 NaH2PO4, 3 KCl, 5 MgSO4, 0.5 CaCl2, 10 glucose, and 248 sucrose (equilibrated with 95% O2-5% CO2, pH 7.4). Acute horizontal OB slices (350-µm thick) were cut with a Leica VT1200s vibratome (Zhou et al. 2016). Slices were incubated in oxygenated artificial cerebrospinal fluid (ACSF; in mM): 124 NaCl, 26 NaHCO3, 3 KCl, 1.25 NaH2PO4, 2 MgSO4, 2 CaCl2, and 10 glucose (equilibrated with 95% O2-5% CO2, pH 7.4) at 30°C for 30 min and then at room temperature (23°C) for at least 1 h before use. For recording, slices were transferred to a recording chamber and perfused with ACSF at a rate of 3 mL/min maintained at a temperature of 30°C (Bipolar Temperature Controller, Norfolk, VA). Target cells were observed with a ×40 water-immersion objective using an Olympus BX51W upright microscope equipped for near-infrared differential interference contrast optics (Olympus Optical, Tokoyo, Japan) and fluorescent excitation/barrier filters suitable for visualization of the fluorescent protein constructs.
Electrophysiology.
Whole cell (current and voltage) patch-clamp recordings were performed as previously described (Shao et al. 2009; Zhou et al. 2008, 2018). Briefly, recording pipettes were pulled from thick-wall borosilicate glass with filament (outside diameter: 1.5 mm, inside diameter: 0.75 mm; Sutter Instrument, Novato, CA) in a horizontal pipette puller (model P-97 Flaming/Brown Micropipette puller; Sutter Instrument, Novato, CA). For current-clamp recordings, the internal solution contained (in mM) 120 K-gluconate, 5.5 MgCl2, 0.5 CaCl2, 10 HEPES, 5 EDTA, 3 Na2ATP, 0.3 Na3GTP, 4 phosphocreatine, and 0.1% biocytin (pH 7.3 adjusted with KOH) and for voltage-clamp recordings, it contained (in mM) 115 CsCH3SO4, 5 MgCl2, 10 HEPES, 5 EDTA, 5 QX-314, 3 Na2ATP, 0.3 Na3GTP, 4 phosphocreatine, and 0.1% biocytin (pH 7.3 adjusted with CsOH). Osmolarities for both solutions were in the range 287−295 mOsm. All data were acquired with pCLAMP9 software using a MultiClamp 700A amplifier (Axon Instruments, San Jose, CA), digitized with a DigiData 1322A (Axon Instruments, San Jose, CA). Inhibitory and excitatory postsynaptic current (IPSC and EPSC) were recorded in voltage-clamp mode at different holding potentials [0 mV for IPSC and −80 mV (or −50 mV) for EPSC] to optimize detection of these currents, and the firing rates were recorded in current-clamp mode without injected current.
Electrical and optical stimulations.
Electrical stimulation of olfactory nerve axons was delivered by bipolar glass electrodes made from theta borosilicate tubes (Sutter Instrument, Novato, CA). Isolated, constant current pulses (170-µs duration, intensity range of 15–45 µA) were triggered by a PG4000A Digital Stimulator (Cygnus Technologies, Southport, NC). Optical stimuli (2 ms) were delivered from a 25-µm multimode optical fiber (0.1 numerical aperture, 7° beam spread; ThorLabs, Newton, NJ) coupled to a 150-mW and 473-nm diode-pumped, solid-state laser (LWBL473083272) gated with a Uniblitz shutter. This shutter gated short-duration optical exposures with paired stimulations at 0.83, 1.25, 2.5, 5, 10, and 20 Hz. ChR2 has kinetic difficulties in following frequencies of 40 Hz or greater, at which rate steady-state plateau currents emerge because the ChR2 current from one stimulation has incompletely returned to baseline by the time the second stimulation has occurred. This limited frequency rates to 20 Hz or less. Optical power delivered at the fiber tip was calibrated with a PM20A Power Meter (ThorLabs, Newton, NJ; delivered power ranges of 0.6–8 mW). The onset and duration of optical stimulation were measured by splitting 1% of the laser beam out to a high-speed (30-ns rise time) silicon photosensor (model 818-BB, Newport, Irvine, CA) and were recorded by the same MultiClamp 700A amplifier as the patch electrode.
Histology.
After electrophysiological recordings, we performed staining as described previously (Zhou and Roper 2011). Briefly, slices with biocytin-filled cells were fixed in 4% paraformaldehyde overnight. Slices were incubated with 4 µg/mL Alexa Fluor 546 streptavidin for 2 h and then DAPI (300 nM) for 1 h. Slices were then mounted on glass slides and imaged using a FluoView500 confocal microscope (Olympus, Tokyo, Japan).
Experimental design and statistical analysis.
MCs, SACs, and PGCs were whole cell recorded for postsynaptic currents and firing rates with or without electric and optical stimulation at different stimulation frequencies. Data were analyzed with Clampfit 10.6 (Molecular Devices, Axon Instruments, San Jose, CA). Statistical analysis and graphical presentation were performed with Origin 2018 (Originlab Corporation, Northampton, MA). Statistical significance of population responses was calculated using Student’s t test or ANOVA with Bonferroni post hoc test depending on statistical degree of freedom in the specific experiment. We obtained paired-pulse ratio (PPR), calculated as the ratio of peak amplitude of the test (second) pulse-evoked response (IPSC or EPSC) to that of control (first) pulse. We estimated the onset synaptic latency of optical stimulation-evoked IPSC as time from the onset of light to the onset of the IPSC, and onset synaptic jitter as the standard deviation of latencies from 20 trials.
Drugs and chemicals.
d-2-amino-5-phosphonovalerate [APV; N-methyl-d-aspartate (NMDA) receptor antagonist, 50 µM), 1,2,3,4-Tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide [NBQX; α-amino-3-hydroxy-5-methylisoxazole propionic acid (AMPA) and kainate receptor antagonist, 10 µM)], gabazine (selective GABAA receptor antagonist, 10 µM), SKF83566 (selective D1-receptor antagonist, 10 µM), and CGP55845 (selective GABAB receptor antagonist, 10 µM) were purchased from Tocris Cookson (Ellisville, MO). All other chemicals including sulpiride (selective D2-receptor antagonist, 100 µM) were purchased from Sigma-Aldrich (Cleveland, OH). All drugs were bath applied by diluting in ACSF at the above-indicated concentration unless otherwise stated.
RESULTS
Differential short-term plasticity of glomerular inhibitory synapses.
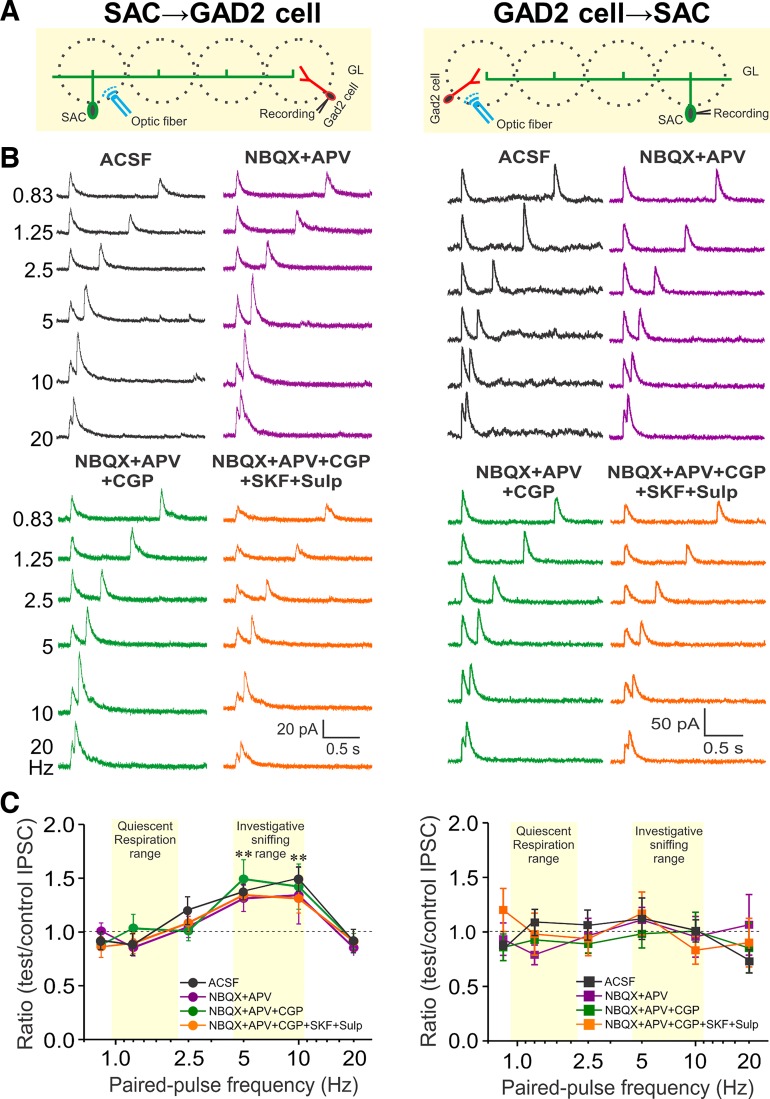
Electrophysiological (Parsa et al. 2015) and anatomical studies (Price and Powell 1970; Toida et al. 1994) report inhibitory-inhibitory interactions within the glomerular circuitry, but the cell types and dynamics are unknown. In the present study, TH-cre mice were crossed with GAD2-GFP+ mice and the olfactory bulbs of the offspring were injected with ChR2-mCherry AAV, resulting in mice with TH+-SAC-ChR2 cells and GAD2-GFP+ cells. Activation of TH+SACs from at least three glomeruli distant (> 200 µm) evoked robust short latency and low-jitter (latency < 2 ms, jitter < 120 µs) IPSCs consistent with monosynaptic input in all recorded GAD2 GFP+ cells (Fig. 1, A and B, left). IPSCs were completely blocked by the GABAA-receptor blocker, gabazine (not shown, n = 5), consistent with our recent study (Shao et al. 2019).
Fig. 1.
Differential short-term plasticity at reciprocal inhibitory synapses between short axon cells (SACs) and glutamic acid decarboxylase (GAD)2-expressing cells. A: schematic diagram showing the experimental design of optical stimulation of channelrhodopsin-2 (ChR2)-SACs (left) or GAD2 cells (right) and recording from GAD2 cells (left) or SACs (right). B: voltage-clamp recording from a GAD2 cell (left) or SAC (right) held at 0 mV in response to paired-pulse light stimulation at 6 different frequencies (0.83, 1.25, 2.5, 5, 10, and 20 Hz) in artificial cerebrospinal fluid (ACSF; black), 1,2,3,4-Tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide (NBQX) + d-2-amino-5-phosphonovalerate (APV) (purple), NBQX+APV+CGP55845 (CGP) (10 µM, green), and further addition of D1/D2 blockers [10 µM SKF83566 (SKF) and 100 µM sulpiride (Sulp)] (NBQX+APV+CGP+SKF+Sulp, orange). C: group data showing paired-pulse ratio of light-evoked inhibitory postsynaptic current (IPSC) peak amplitude of the test pulse to the control pulse at 6 different stimulation frequencies from 5 periglomerular cells and 8 SACs in ACSF and different treatments. **P < 0.01.
To assess whether there is short-term potentiation (STP) or depression (STD) at this inhibitory synapse, we used paired-pulse optical activation of SACs at frequencies (0.83–20 Hz) spanning across the range of basal respiration (~1.5 Hz) to maximal sniff rate (~10 Hz; Wachowiak 2011). In all paired-pulse experiments, interstimulus intervals were delivered in random order to obviate long-term facilitation/depression-like effects. STP was assessed as the paired-pulse ratio (PPR) of the test synaptic peak amplitude to the control. There was pronounced paired-pulse facilitation (PPF) at the SAC→GAD2 cell synapse for activation frequencies of 5–10 Hz (PPR > 1; Fig. 1, B and C, left; P < 0.05; n = 5) but not at lower frequencies (0.83, 1.25, 2.5 Hz) or at the highest frequency tested (20 Hz). SACs inhibit ETCs (Liu et al. 2013), which might reduce excitation of GAD2 cells. Thus, the experiments were repeated with NBQX+APV to block excitation by ETCs. The control IPSC peak amplitudes (~35 pA in ACSF and in NBQX+APV, n = 8, Fig. 1B, left) and PPRs (Fig. 1, B and C, left; n = 5) were unchanged. These findings show that SACs directly inhibit GAD2 cells by activating GABAA receptors and that SAC→PGC inhibition facilitates (at 5–10 Hz) a range of activation frequencies similar to investigatory sniffing rates.
Next, we asked whether the GAD2 cell to TH-SAC synapse also supports STP. For this we crossed GAD2-cre mice with mice where GFP is under control of TH promoter. ChR2-mCherry AAV was injected into olfactory bulbs of the offspring to obtain GAD2-ChR2 cells and GFP+TH cells. Although GAD2 is expressed by ~20% of SACs (Parrish-Aungst et al. 2007), the results at the GAD2 cell→SAC synapse suggest that the net effect of this potential confound is minimal (see below and discussion). Optical activation of GAD2-ChR2 cells evoked a robust IPSC in all recorded TH-GFP+ SACs (Fig. 1, A and B, right). The latency (<2 ms) and jitter (<200 µs) of the IPSCs were consistent with a monosynaptic response, as previously reported (Shao et al. 2019). These IPSCs were completely abolished by gabazine (not shown, n = 5) and unchanged when circuit excitatory actions were blocked by NBQX+APV (control IPSC peak amplitude ~30 pA in ACSF and in NBQX+APV, Fig. 1, B and C, n = 5), consistent with our recent study (Shao et al. 2019). To investigate STP/STD at this synapse, we used paired-pulse optical stimulation (0.83–20 Hz) to activate GAD2 cell→SAC synapses and the PPRs calculated as above. Interestingly, unlike SAC→GAD2 cells, the PPRs in GAD2 cells→SAC were unchanged at all frequencies from 0.83–20 Hz (PPR, ~1.0; Fig. 1, B and C, right; n = 8). This indicates that GAD2 cell synaptic inhibition to SACs is frequency independent.
GAD2 cells release GABA and SACs corelease GABA and DA (Liu et al. 2013). DA is tonically released and acts at pre- and postsynaptic sites to modulate glomerular circuitry (Karpuk and Hayar 2008; Liu et al. 2013; McGann et al. 2005; Vučinić et al. 2006); either or both could modulate IPSC amplitudes and/or PPR at glomerular inhibitory synapses. Addition of CGP55845 (10 µM) to block GABAB receptors had no effect on either PPRs or IPSC amplitudes of either SAC→GAD2 and GAD2 cell→SAC synapses (Fig. 1, B and C; n = 5 for SAC-GAD2 cell and n = 8 for GAD2 cell-SAC synapse). To investigate DA’s role at SAC→GAD2 synapse, the selective D1 and D2 receptor antagonists (10 µM SKF83566, 100 µM sulpiride) were added to NBQX+APV+CGP55845. This reduced both control and test IPSC peak amplitudes by 43% (22.27 ± 2.16 pA in ACSF and 12.73 ± 1.14 pA in blockers; P < 0.01, n = 5) (Shao et al. 2019) but had no effect on PPR (Fig. 1, B and C, left; n = 5). Similarly, at the GAD2→SAC synapse both the control and test IPSC amplitudes were reduced by 23% in D1/D2 blockers (48.6 ± 11.7 pA in ACSF and 37.4 ± 11.7 pA in blockers, P < 0.05; n = 8) (Shao et al. 2019), but there was no impact on GAD2→SAC PPRs (Fig. 1, B and C, right; n = 8).
STP enhances inhibition of output neurons.
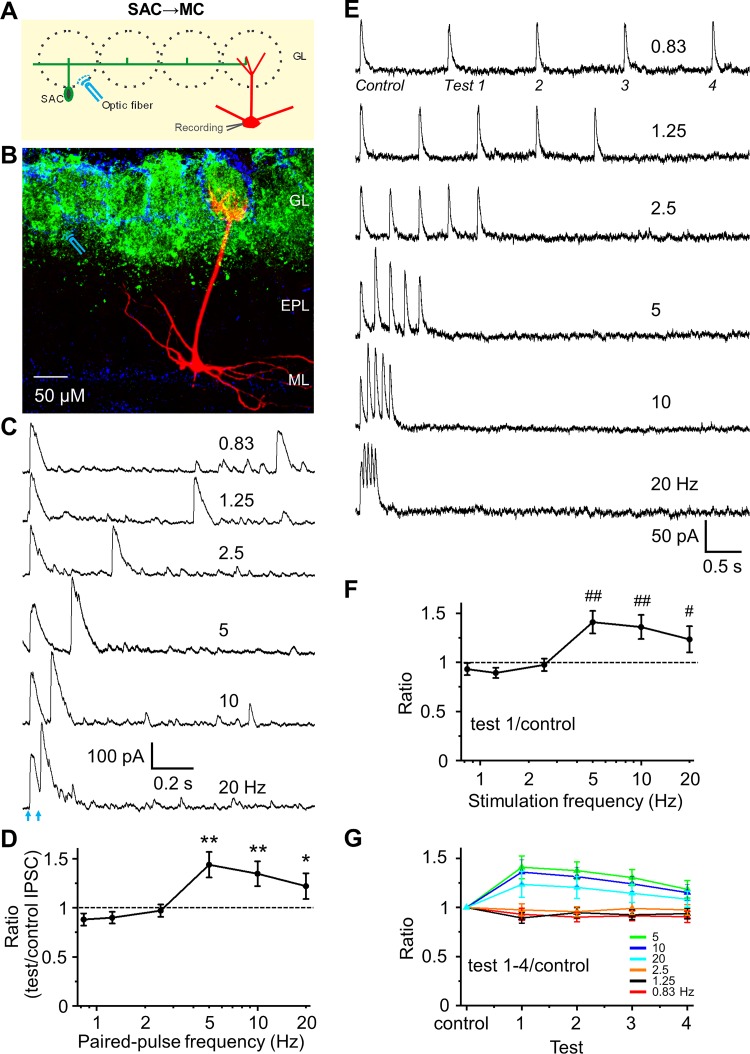
SACs form the interglomerular circuit, which inhibits MC and ETCs in distant glomeruli (Aungst et al. 2003; Banerjee et al. 2015; Borisovska et al. 2013; Kiyokage et al. 2010; Liu et al. 2013, 2016; Vaaga et al. 2017; Whitesell et al. 2013). MC intrinsic membrane properties prolong the duration of SAC inhibitory inputs (Liu et al. 2016). Does STP of the SAC→MC synapse further enhance inhibition of MCs? Selective optical activation of SACs from 3–6 glomeruli distant (200–600 µm) consistently evoked IPSCs in MCs (Fig. 2, A–C), as expected from previous studies (Liu et al. 2016). Although there was no STP at low frequencies (0.83, 1.25, and 2.5 Hz), there was pronounced paired-pulse facilitation at 5, 10, and 20 Hz (n = 7; Fig. 2, C and D). The peak amplitude, 10–90% rise time, and 90–10% decay time constant of IPSCs at the SAC-MC synapse (at 0.83 Hz) were 275.6 ± 41.9 pA, 2.07 ± 0.26 ms, and 46.57 ± 8.94 ms (n = 7), respectively.
Fig. 2.
Frequency-dependent short-term facilitation of synapses from short axon cells (SACs) to mitral cells (MCs). A: schematic diagram showing the experimental design of optical stimulation of channelrhodopsin-2 (ChR2)-SACs and recording from MCs. B: labeling of ChR2 in SACs (green), DAPI (blue), and biocytin-filled MC (red). Optical stimulation of ChR2-SACs was performed at ~300 µm distance from the apical dendrite of voltage clamp-recorded MC. C: example traces of optical stimulation (arrows)-evoked inhibitory postsynaptic currents (IPSCs) from a MC held at 0 mV in response to paired-pulse optical stimulation at 6 different frequencies (0.83, 1.25, 2.5, 5, 10, and 20 Hz) in artificial cerebrospinal fluid (ACSF). D: group data showing paired-pulse ratio (PPR) of evoked IPSC peak amplitude of the test pulse to the control pulse at different frequencies in ACSF. Optical stimulation induces paired-pulse facilitation at frequency of 5, 10, and 20 Hz, with a significantly larger ratio than 1. E: example traces of a train of 5-pulse optical stimulation-evoked IPSCs from another cell. F: population data for ratio of IPSC peak amplitude of the test pulse 1 to control, evoking PPR equivalent to the paired pulse experiment in D. G: population data for ratio of IPSC peak amplitude of the test pulses 1–4 to control. A train of 5-pulse optical simulation-induced short-term synaptic facilitation of each IPSC in the sequence with a progressive decline in magnitude with continued stimulations in the train. **P < 0.01, *P < 0.05, ##P < 0.01, and #P < 0.05 vs. PPR = 1; n = 7 for D and n = 5 for F and G. EPL, external plexiform layer; GL, glomerular layer; ML, mitral cell layer.
Animals display brief bouts of high-frequency (5–10 Hz) sniffing when sampling odors. The number of sniffs present in a bout of investigatory sniffing behavior ranges from 1–5, with the possibility of 1 sniff per bout ~46%, 2 per bout ~20%, and 5 or more only ~20% in ice (Sirotin et al. 2014). Indeed, the odor presence and strength can be determined in just one or two sniffs depending on the complexity of the discrimination task (Mainland and Sobel 2006; Uchida and Mainen 2003). Although paired-pulse approaches recapitulate the majority of inputs in a behavioral bout, we also tested whether a train of 5-pulse stimulations at different frequencies impacted STP (Fig. 2, E–G). The data show that a train of 5-pulse optical simulation induced a short-term synaptic facilitation of IPSCs to pulses 2–5 at frequencies of 5–20 Hz, similar to the paired-pulse facilitation. A progressive diminishment in the magnitude of STP did occur with successive stimulations in the train (Fig. 2, E–G).
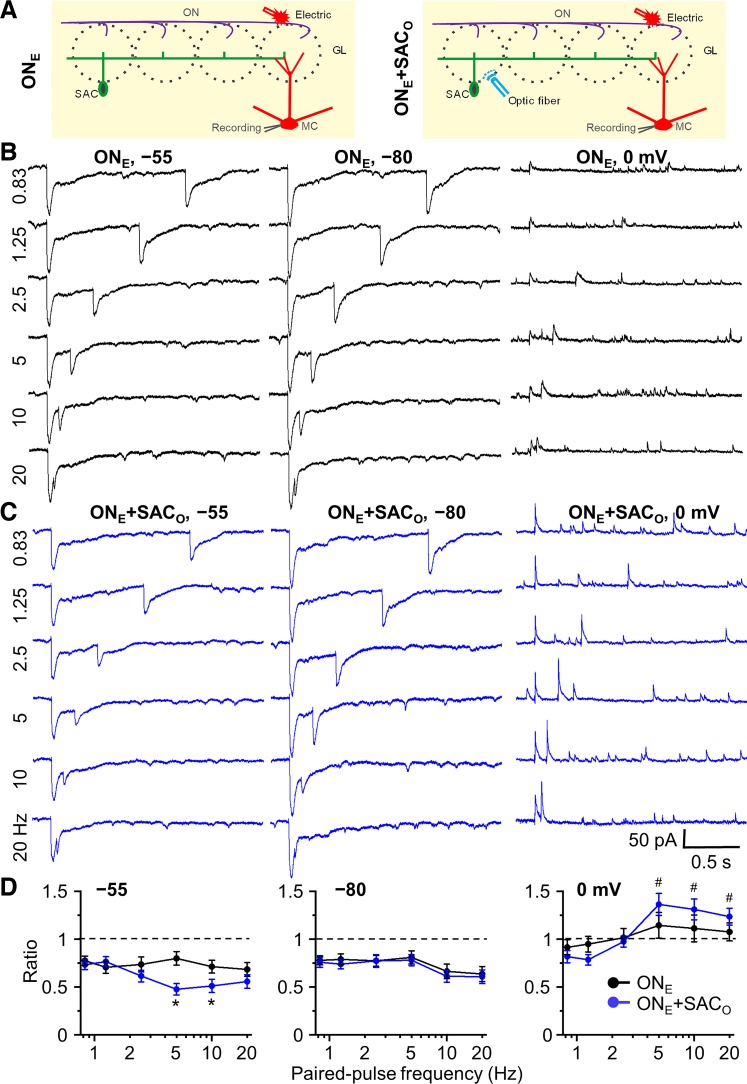
Does frequency-dependent STP alter MC’s response to olfactory nerve (ON) input? To test this, the ON was activated by electrical stimulation applied rostral to the glomerulus containing dendrite of a recorded MC (Fig. 3A). Each cell was held at –55, –80, and 0 mV and stimulated at different paired-pulse stimulation frequencies (0.83, 1.25, 2.5, 5, 10, and 20 Hz) with electrical and electrical combined with optical stimulation. To observe facilitation or depression, the strength of optical and electric stimulation was empirically adjusted to 50% of the intensity necessary to elicit a maximal optically evoked IPSC (at holding potential of 0 mV, optical power range 0.6–4 mW) or electrically evoked EPSC (at holding potential of −55 mV, stimulation intensity 15–45 µA), respectively.
Fig. 3.
Impact of frequency-dependent short-term plasticity of short axon cell (SAC) inhibition on olfactory nerve electrical stimulation-induced synaptic currents in mitral cells (MCs). A: schematic diagram showing the experimental design of electric stimulation of olfactory nerve (ONE, left) and both ONE and optical stimulation of channelrhodopsin-2 (ChR2)-SACs (ONE+SACO, right). B: MC currents were recorded at 3 different holding potentials: −55 mV (~MC resting membrane potential; Liu and Shipley 2008), −80 mV [to isolate excitatory postsynaptic currents (EPSCs) with minimal Cl− driving force for inhibitory postsynaptic currents (IPSCs)], and 0 mV (to isolate IPSCs by enhancing Cl− driving force and minimizing EPSCs). Example traces of paired-pulse electric stimulation-induced EPSCs (inward current) at the holding potential of −55 and −80 mV, and IPSCs (outward current) at 0 mV with different paired-pulse frequencies of 0.83, 1.25, 2.5, 5, 10, and 20 Hz. C: example traces of ONE+SACO-induced EPSCs and IPSCs at different holding potentials with different paired-pulse frequencies. D: group data for paired-pulse ratio of postsynaptic currents of the test pulse to the control at the holding potentials of −55, −80, and 0 mV with different paired-pulse frequencies. ONE and ONE+SACO at different frequencies consistently induced paired-pulse depression of EPSCs at holding potential of −55 and −80 mV. ONE+SACO significantly increased paired-pulse depression at holding potential −55 mV at frequency of 5 and 10 Hz but did not enhance the depression at −80 mV at all frequencies. In contrast, at holding potential of 0 mV, ONE+SACO induced paired-pulse facilitation at 5, 10, and 20 Hz; ONE alone did not induce significant paired-pulse plasticity. The strength of optical and electric stimulation was adjusted to 50% maximal IPSC (at holding potential of 0 mV) and EPSC (−55 mV), respectively, so that either enhanced or suppressed IPSCs/EPSCs could be observed. Optical stimulation: 0.6–4 mW; electric stimulation: 15–45 µA. *P < 0.01 vs. ONE and #P < 0.01 vs. ratio of 1; n = 6. GL, glomerular layer.
At –55 mV, ON stimulation evokes partially overlapping inward and outward currents resulting in net excitation (De Saint Jan et al. 2009; Gire et al. 2012; Hayar et al. 2004b). Paired-pulse stimulation of the ON, alone, caused short-term, frequency-independent depression (PPR = ~0.75, Fig. 3, B–D) corresponding to presynaptic inhibition of ON terminals (Aroniadou-Anderjaska et al. 2000; Ennis et al. 2001; Karpuk and Hayar 2008; McGann et al. 2005; Murphy et al. 2004; Vaaga et al. 2017; Vučinić et al. 2006; Wachowiak and Cohen 1999). When paired with optical activation of SACs, the amplitude of the ON-evoked EPSC was reduced. Subtraction of the EPSC to ON plus SAC activation (ONE+SACO) from the control ON stimulation alone EPSC (ONE) indicates that the net inhibition from SAC activation at –55 mV was 49.4 ± 8.1 pA [ONE–(ONE+SACO) at 0.83 Hz, n = 6, Fig. 3, B and C]. This net inhibitory effect at –55 mV was smaller than the IPSC peak amplitude of 275.6 ± 41.9 pA at 0 mV (0.83 Hz, n = 7, Fig. 2C) due to higher Cl− driving force at that potential.
At ON-SAC paired stimulation frequencies on 0.83, 1.0, and 2.5 Hz, the PPRs were the same as ON stimulation alone (~0.75, P > 0.05; Fig. 3, B–D). However, at frequencies of 5 and 10 Hz, there was pronounced additional suppression of the test EPSC (PPR = 0.475 ± 0.059 at 5 Hz and 0.511 ± 0.068 at 10 Hz; P < 0.01 vs. ONE at 5 and 10 Hz, respectively; n = 6, Fig. 3, B and C). If this is due to STP at SAC synapses, then when the MC is held at –80 mV (to minimize IPSC driving force) there should be no difference between ON stimulation alone and when combined with SAC activation. Indeed, at –80 mV, the PPR was identical between ON only and ON plus SAC stimulation (Fig. 3, B–D). Finally, at 0-mV holding potential to minimize EPSCs and unmask the IPSC, ON stimulation alone evoked a small IPSC (Fig. 3B). Paired-pulse ON stimulation exhibited no STP at low frequency (0.83, 1.25, 2.5 Hz; Fig. 3, B and D) and slight facilitation at 5–10 Hz. However, when ON stimulation was paired with SAC activation, there was pronounced PPF at 5–20 Hz (Fig. 3, C and D). Thus, MC excitability decreases as SAC-evoked synaptic stimulation frequency increases. This excitability decrease could be direct hyperpolarization from the increased IPSC in MCs and/or conductance changes in the MC associated with GABA shunt.
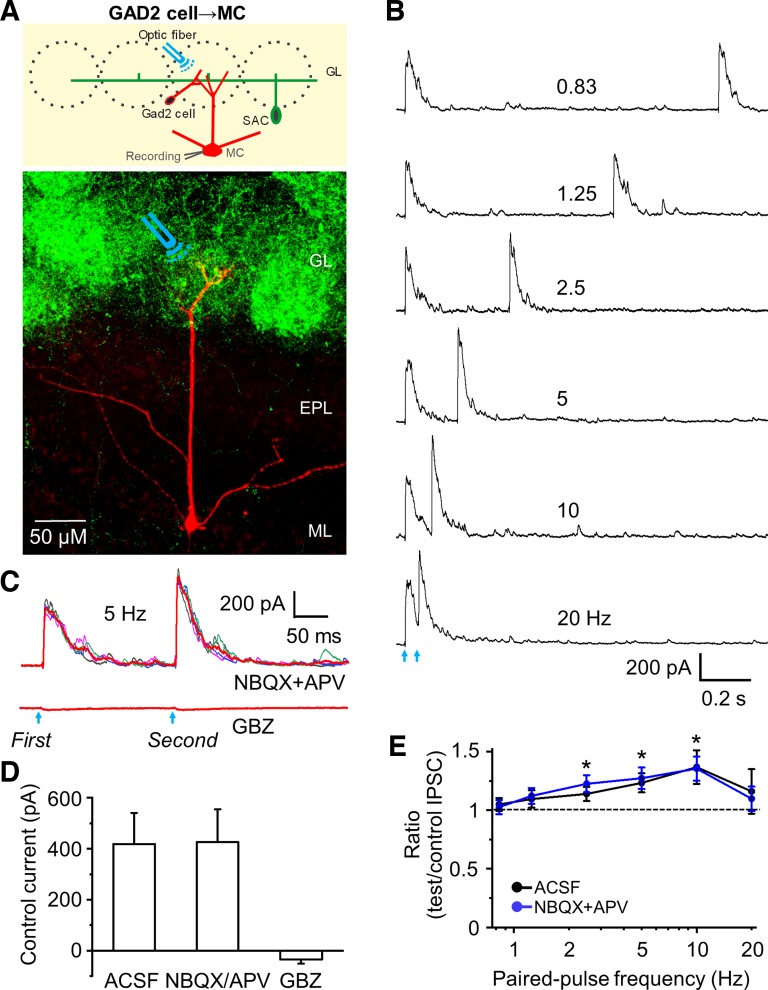
GAD2 cell→SAC inhibition is frequency independent. Is this also true of the GAD2 cell→MC inhibitory synapse? To assess this, we activated GAD2-ChR2 neurons in a glomerulus containing the apical dendrite of a recorded MC (Fig. 4A). Activation of GAD2 cells evoked outward current (latency 2.49 ± 0.15 ms and jitter 167 ± 41 µs; n = 10, Fig. 4B) indicative of GAD2 cell monosynaptic inhibition of MCs. Bath application of both NBQX and APV (n = 7) had no effect on the IPSC, ruling out excitatory circuit effects. The outward current was blocked completely by gabazine (n = 10, Fig. 4, C and D). Next, we recorded IPSCs evoked by paired-pulse activation of GAD2 cells. As activation frequency increased from 0.83 to 20 Hz (n = 10; Fig. 4E), PPRs increased steadily to a maximum of 1.37 ± 0.14 at 10 Hz. NBQX and APV had no effect on this PPF, ruling out excitatory circuit contributions (Fig. 4E). The peak amplitude, 10–90% rise time, and 90–10% decay time constant of control IPSCs at the GAD2 cell-MC synapse (at 0.83 Hz) were 418.44 ± 122.33 pA, 2.21 ± 0.24 ms, and 74.4 ± 11.2 ms (n = 7), respectively. The peak amplitude and decay time were significantly larger than those at SAC-MC synapses (P < 0.01); however, the rise time of IPSCs at the GAD2 cell-MC synapse was not significantly different from the SAC-GAD2 synapse (P > 0.05). Taken with the findings above, we conclude that GAD2 cells→MC synapses support STP, whereas GAD2→SACs synapses do not.
Fig. 4.
Activation of channelrhodopsin-2 (ChR2)-glutamic acid decarboxylase (GAD)2 cells evokes paired-pulse facilitation of inhibitory postsynaptic currents (IPSCs) in mitral cells (MCs). A: schematic diagram showing the experimental design of optical stimulation of ChR2-GAD2 cells and recording from MC (upper) as well as labeling of ChR2 in GAD2 cells and their dense dendrites (green) and biocytin-filled MC (red) (bottom). Optical stimulation of ChR2-GAD2 cells that synapse with apical dendritic tufts of MC was performed. B: example traces showing evoked IPSCs in artificial cerebrospinal fluid (ACSF) in MC at holding potential of 0 mV in response to paired-pulse optical stimulation at 6 different frequencies. C: example of 4 individual traces and the averaged trace (red) of synaptic currents at stimulation of 5 Hz in 1,2,3,4-Tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide (NBQX; 10 µM) + d-2-amino-5-phosphonovalerate (APV; 50 µM), and gabazine (GBZ; 10 µM). D: group data showing the peak amplitude of optical stimulation-evoked IPSCs to the control pulse in ACSF (n = 10), NBQX+APV (n = 7), and GBZ (n = 10). Combination of NBQX and APV did not change the peak amplitude of IPSC to control and test pulse. Neither individual NBQX nor APV changed the peak amplitude (both n = 3, not shown). GBZ completely blocked IPSCs. E: group data showing paired-pulse ratio of optical stimulation-evoked IPSC peak amplitude of the test pulse to the control in ACSF (n = 10) and NBQX+APV (n = 7). Paired-pulse facilitation was observed at 2.5, 5, and 10 Hz with ratios significantly larger than 1, and the ratios increased with increasing stimulation frequency from 0.83 to 10 Hz. *P < 0.01 vs. paired-pulse ratio of 1. EPL, external plexiform layer; GL, glomerular layer; ML, mitral cell layer.
STP at SAC→MC synapses suppresses MC spike output.
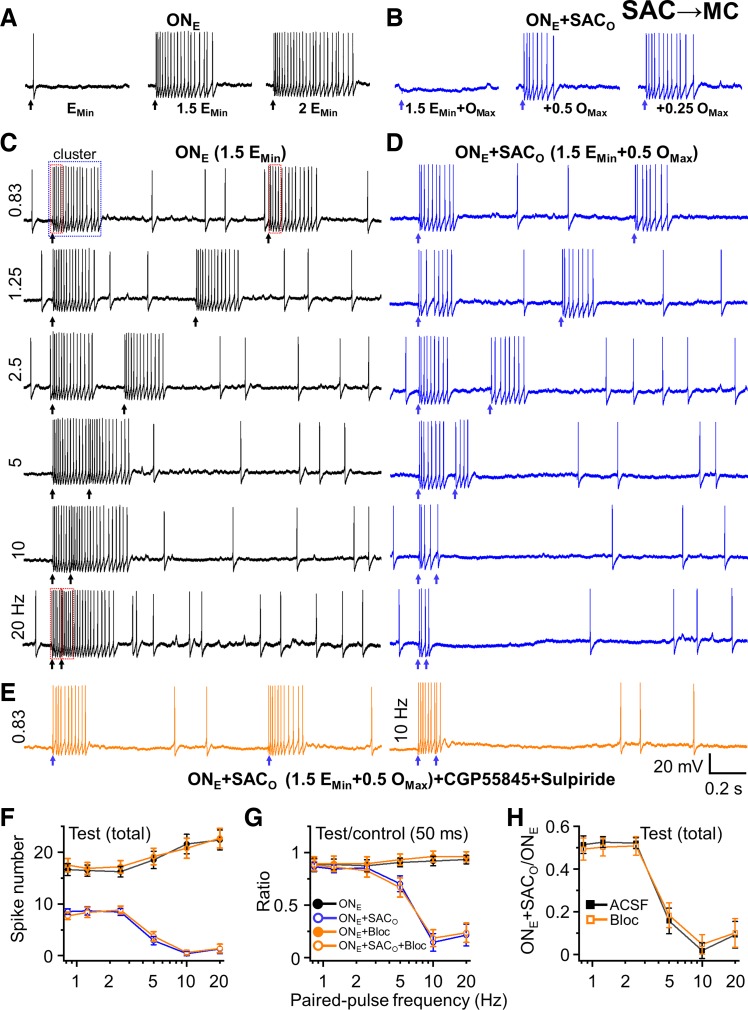
Stimulation of the ON alone evoked MC spike output proportional to the intensity of the stimulating current (Fig. 5A). Intensity was adjusted for each cell to evoke ~50% maximal spiking so that enhanced or suppressed spiking could be observed (Fig. 5A). Strong optical activation of SACs completely inhibits ON-evoked MC spiking (Liu et al. 2016). The laser power used to activate SACs was adjusted to a level halfway between complete inhibition and no inhibition of MC spiking (Fig. 5B). ON stimulation alone at low frequency (0.83, 1.25, and 2.5 Hz) elicited an epoch of spiking (17.38 ± 0.74 spikes at 0.83 Hz), and a test/second ON stimulation elicited a similar number of spikes (16.65 ± 0.63 spikes at 0.83 Hz, n = 7). Interestingly, although there was paired pulse suppression of the EPSC (PPR ~0.75; Fig. 3), there was no difference in spiking between the control and test stimulation at 0.83, 1.0, or 2.5 Hz. One possible explanation is that the impact of paired-pulse depression of ON terminals at the intensity used may not be strong enough to influence MC firing differentially between test and control pulses. This is consistent with the narrow range of ON stimulation intensity between no response in a MC and full response (Carlson et al. 2000). At higher ON stimulation frequencies (5–20 Hz), spiking evoked by the control ON stimulation had not terminated when the test stimulation occurred; thus, the spike number evoked by the control stimulation was truncated by the test stimulation. The number of spikes evoked by the test stimulation was slightly greater at high versus low ON frequencies (18.51 ± 1.67 spikes at 5 Hz, 21.58 ± 1.86 spikes at 10 Hz, and 22.37 ± 1.91 spikes at 20 Hz, Fig. 5F, n = 7). Expressed as ratios, the PPRs were unchanged at ON stimulation from 0.83 Hz (PPR ~1.0, n = 7; Fig. 5G). When combined with optical stimulation of SACs, ON-evoked MC spiking to the control and test stimulation was reduced by 50% at low frequencies (Fig. 5, D and F). This was expected because the optical stimulation intensity was adjusted to give 50% reduction to single ON shocks. The PPRs were unchanged at lower frequencies (0.83, 1.25, 2.5 Hz). However, at higher frequencies the PPRs were dramatically suppressed (5-Hz PPR = 0.704 ± 0.075; 10-Hz PPR = 0.147 ± 0.084; 20-Hz PPR = 0.215 ± 0.103; Fig. 5G).
Fig. 5.
Impact of paired-pulse plasticity of short axon cell (SAC) to mitral cell (MC) synapses on MC spiking. A: spike number of firing cluster increases with increasing intensity of electric stimulation from minimum intensity (EMin; operationally defined in multiples of the minimum current required to elicit a spike in each recorded MC, 20 µA in this cell, range: 10–25 µA in all cells), 1.5 times EMin (eliciting ~50% of maximal spiking), to twofold of EMin (2 EMin, eliciting maximal MC spiking). B: optical stimulation completely or partially reverses electric stimulation (1.5 EMin)-induced firing. Maximum optical stimulation (OMax,) is operationally defined as the fraction of the optical stimulation intensity that abolished all spiking elicited by 1.5 EMin in each recorded MC (1.8 mW in this cell, range: 1.3–4.0 mW in all cells). Lower optical stimulations, 0.5 OMax (0.9 mW) and 0.25 OMax (0.45 mW), induce partial inhibition of electric stimulation-induced firing. C: paired-pulse electric stimulation (1.5 EMin) does not induce paired-pulse plasticity with a similar spike number of firing cluster to control and test pulse up to 5 Hz, with a modest enhancement of spike number to the test pulse present at 5, 10, and 20 Hz. D: paired optical stimulation (0.5 OMax) induced an inhibition of 1.5 EMin-induced firing cluster to test and control pulse at all frequencies. As frequency increases above 5 Hz, the magnitude of optical suppression of spiking in the test stimulation is enhanced, whereas there is no enhancement at frequencies of 2.5 Hz or less. E: blockade of GABAB and D2 receptor (10 µM CGP55845+100 µM sulpiride) did not change the inhibition of SACs in MC spiking. Example traces show similar spike number of firing cluster in test pulse to control at example frequency of 0.83 Hz, but much less spike number in test pulse to control at 10 Hz. F: total spike number of firing cluster to test pulse after stimulation shows that electric stimulation induces similar spike number of firing cluster at different stimulation frequencies from 0.83–20 Hz, but optical stimulation partially inhibits 1.5 EMin electric stimulation-induced firing cluster with less spike number at higher stimulation frequencies of 5, 10, and 20 Hz compared to lower stimulation frequencies (0.83, 1.25, and 2.5 Hz). G: group data showing ratios (spike number of firing cluster to test pulse/control pulse) much less than 1 (‘paired-pulse depression’) at 5, 10, and 20 Hz with both electric and optical stimulation (ONE+SACO) but not electric stimulation alone (ONE). Blockade of GABAB and D2 receptor (Bloc, 10 µM CGP55845+100 µM sulpiride) did not change the ratios. To obtain ratios, the stimulation-induced 250–300 ms firing cluster (blue dot box in C) and the spike numbers in a 50-ms poststimulation time window (red dot boxes in C) were assessed. This window was chosen at 20 Hz because there is only a 50-ms interstimulus interval in total. To validate this approach, we compared the first 50-ms window against a total spiking epoch for the low frequencies of 0.83, 1.25, and 2.5 Hz, which have sufficient interstimulus interval to do so. The ratio of spike number from either whole firing cluster or only partial 50-ms window immediately after stimulations was similar. This indicated that the analysis of a 50-ms window provides a reasonable comparison for the spiking ratio of the paired-pulse events across the entire frequency range. Legends in G apply to F. H: ratio of total spike number of firing cluster to the test/second pulse with both electric stimulation and optical stimulation to electric stimulation alone (ONE+SACO/ONE) also shows that optical stimulation at higher frequencies (5, 10, and 20 Hz) induces a firing cluster with much less spike number than at low frequencies (0.83, 1.25, and 2.5 Hz) that was not changed by blockade of GABAB and D2 receptor (‘Bloc’). n = 7 for artificial cerebrospinal fluid (ACSF) and n = 6 for Bloc in F, G, and H.
To further assess how STP of SAC synapses reduces MC spike output, we compared the total spikes evoked by the test ON stimulation with coactivation of SACs to that evoked by ON stimulation alone (Fig. 5H). This measures the suppression attributable to activation of SACs. There were no differences at 0.83, 1.25, and 2.5 Hz (ratio = ~0.5), but at 5, 10, and 20 Hz, MC spike ratios were reduced to 0.157 ± 0.059, 0.017 ± 0.037, and 0.092 ± 0.062, respectively (n = 7; Fig. 5H). Thus, STP at the SAC→MC synapse potently inhibits MC responses to sensory input frequencies corresponding to those of investigative sniffing. This suggests that the interglomerular network may increasingly control MC output during high-frequency sniffing (Wachowiak and Shipley 2006).
Finally, we examined whether GABAB and D2 contribute to SAC inhibition of MC spiking. We found that GABAB (CGP55845, 10 µM) and D2 blocker (sulpiride, 100 µM) had no effect on SAC inhibition of ON-evoked MC spiking (Fig. 5, E–H). Together with the GABAB and DA data on SAC↔GAD2 cell synaptic connections described above, these results show that tonic DA release enhances the strength of glomerular inhibition but has little to no role in frequency-dependent STP.
DISCUSSION
Odors are transduced by olfactory sensory neurons whose axons synapse in glomeruli. ON terminals synapse on M/TCs, the principal main olfactory bulb output neuron. This straight-through excitatory pathway is modulated by GABAergic inhibitory interneurons in the glomerular and granule cell layers (Burton et al. 2017; Díaz et al. 2017; Isaacson and Strowbridge 1998). The principal glomerular inhibitory neurons are the GABAergic PGCs and GABAergic/dopaminergic SACs. PGCs are primarily ‘uniglomerular’ and form ‘intraglomerular’ inhibitory circuits within a single glomerulus (Kiyokage et al. 2010; Shao et al. 2012, 2013). SACs synapse with neurons in other glomeruli to form interglomerular circuits (Aungst et al. 2003; Borisovska et al. 2013; Kiyokage et al. 2010; Kosaka and Kosaka 2008; Liu et al. 2013, 2016; Shirley et al. 2010; Whitesell et al. 2013). We have also shown strong inhibitory interactions between PGCs and SACs (Shao et al. 2019).
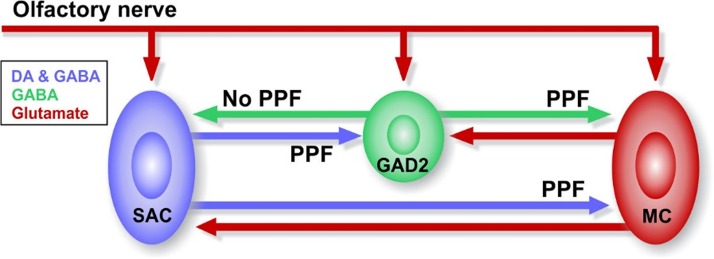
Glomerular circuits are dynamically regulated. Sensory signals from olfactory sensory neurons are episodic based on inhalation-exhalation cycles, which in rodents range from 0.5 Hz in basal respiration to 5–10 Hz during investigatory sniffing (Carey and Wachowiak 2011; Verhagen et al. 2007; Wachowiak 2011; Wesson et al. 2008). During mouse investigatory sniffing, the animal performs 1–5 individual sniffing events during the bout, with the possibility of 1 sniff ~46%, 2 sniffs ~20%, and 5 or more sniffs less than 20% (Sirotin et al. 2014), indicating that the most common investigatory behavior bout contains 1–2 sniffs. The sniffing required for detection of the presence of an odor or a strong odor is typically determined behaviorally in a single sniff (Uchida and Mainen 2003). More complex discrimination of mixtures does require multiple sniffs and a longer bout (Mainland and Sobel 2006). Under more difficult discrimination tasking, we hypothesize that sniff sampling might engage STP of glomerular inhibitory synapses and shape output signals as a function of input frequency. Our results showed that selective components of the glomerular inhibitory circuit are differentially modulated by activation frequencies corresponding to investigatory sniffing (5–10 Hz). PGC→SAC inhibition was frequency independent at all frequencies tested. In contrast, inhibition at SAC→PGC synapses had strong PPF at 5–10 Hz. Both SACs and PGCs inhibited MCs and reduced spike output. These findings show that glomerular inhibitory circuits are dynamically and differentially regulated at frequencies corresponding to investigative sniffing.
Repetitive stimulation of the olfactory nerve induces presynaptic inhibition via dopamine and GABAB receptors, which can reduce the ON-evoked EPSC by ~30% (Aroniadou-Anderjaska et al. 2000; Ennis et al. 2001; Karpuk and Hayar 2008; McGann et al. 2005; Murphy et al. 2004; Vaaga et al. 2017; Vučinić et al. 2006; Wachowiak and Cohen 1999). We observed the same reduction phenomena in voltage clamp with ON electrical stimulation; however, at the intensity used in the current clamp experiments, there was limited change to MC spiking. Our interpretation is that at an electrical stimulation calibrated to result in approximately half-maximal spiking, the reduction in an EPSC from GABAB/DA-mediated presynaptic feedback may be insufficient to significantly change the spiking pattern at this stimulation threshold. Interestingly, although SAC stimulation potently reduced MC spiking as frequency increased, this inhibition was unaltered by GABAB/DA blockers. Thus, stimulation of the interglomerular SAC network does not appear to contribute to presynaptic inhibition in distant glomeruli, although with simultaneous ON/SAC stimulation the pharmacokinetic delay in GABAB/DA makes this paradigm suboptimal for conclusions on presynaptic inhibitory roles. However, this observation is consistent with previous reports that interglomerular circuitry plays no role in presynaptic inhibition of ON terminals (McGann et al. 2005). We also cannot exclude that peri- or subthreshold stimulation intensity might be modulated by SAC-mediated presynaptic inhibition. How could dopamine act presynaptically if experiments do not show a role when SACs are stimulated? SACs have short- and long-range projection subtypes, commonly with a principal glomerulus containing abundant processes (Bywalez et al. 2017; Galliano et al. 2018; Kiyokage et al. 2010). Potentially, if only short-range SACs are involved in dopamine-mediated presynaptic inhibition of the ON, they would not have been stimulated in the configuration of our experiments. Alternatively, dopamine release to the ON might occur through the principal glomeruli processes close to the cell body and not from terminals in distant glomeruli. We have observed that as the stimulation frequency increases, both SAC drive onto MCs and onto GAD2 cells increases. It is possible that increased drive onto GAD2 cells may decrease GAD2 cell function and, in turn, decrease GAD2 cell GABA release to the olfactory nerve, thereby decreasing presynaptic inhibition as the SAC network becomes potentiated. These possibilities remain to be tested experimentally.
Tyrosine hydroxylase is a selective marker for the dopaminergic SACs; however, although GAD2 is expressed by PGCs, there are some SACs that also express GAD2 (< 20%) (Parrish-Aungst et al. 2007). Thus, some GAD2-expressing SACs may also be engaged by activation of GAD2-ChR2 cells. However, whereas SAC synapses to both PGCs and MCs had robust STP, GAD2 cells only had STP at synapses with MCs and not with SACs. The markedly different STP profiles of the two cell types suggest that ‘contamination’ by GAD2-expressing SACs is minor. Why might this be? GAD2-expressing SACs may be too few in number to contribute measurably to the STP profile of the GAD2-ChR2 cells. Alternatively, there are long- and short-range projecting SACs (Galliano et al. 2018; Kiyokage et al. 2010). If GAD2-expressing SACs were preferentially short-range cells, they may not have been activated by our optical fibers that were placed at a distance from the glomerulus containing the recorded MC or PGC. Finally, GAD2 could be expressed transiently in SACs. Expression before maturation is observed in stem cells of the olfactory bulb throughout life, with immature DA cells expressing transgene/mRNA before expression of functional proteins (Baker et al. 2001; De Marchis et al. 2004; De Marchis et al. 2007; Galliano et al. 2018; Kohwi et al. 2005; Lois and Alvarez-Buylla 1994; Luskin 1993; Plachez and Puche 2012). The possible mechanism remains to be determined; however, we are able to conclude that GAD2 cell inhibition (PGCs) from GAD2-expressing SACs is functionally limited.
Why might SACs and PGCs have different STP profiles? There are several possibilities. PGCs may contact both MCs and SACs, but the mechanism for STP is only present at PGC→MC synapses. Such differential synaptic kinetics in the same neuron have been reported for cell connections in cortex and hippocampus (English et al. 2017; Matveev and Wang 2000). Alternatively, PGCs are a heterogenous population of inhibitory neurons with subpopulations classically defined by calcium-binding protein expression (Benito et al. 2018; Kosaka et al. 1998; Nagayama et al. 2014; Najac et al. 2015; Parrish-Aungst et al. 2007; Philpot et al. 1997). Other studies have demonstrated two types of PGCs: ~30% receive monosynaptic ON input, whereas ~70% are polysynaptically activated by ON (Shao et al. 2009). Thus, it is possible there are different subtypes of PGCs, with those exhibiting short-term facilitating synapses selectively targeting MCs and those with nonfacilitating synapses targeting SACs. If this is the case, the absence of short-term facilitation at the PGC to SAC synapse itself could be the net effect of PGCs that facilitate in combination with PGCs that show depression, yielding a net absence of detectable STP. Isolating the synaptic properties and interconnectivity of each subpopulation of PGCs and SACs may shed light on these possibilities.
We also observed PPF of SAC to GAD2 cell synapse and at the GAD2 cell to MC synapse to stimulation frequencies of 5–10 Hz but the absence of facilitation at 20 Hz. This could be due to a decrease in the number of readily releasable pool (RRP) of vesicles rate, limiting transmission efficacy at this highest frequency. The number of synaptic vesicles released depends on the size of RRP and the initial probability of release (Fioravante and Regehr 2011). PPF is commonly indicative of a low initial probability of release, such that additional vesicles are ‘primed’ by the first stimulation and available in larger amounts to a second stimulus (Thanawala and Regehr 2016). When release probability is high at higher-frequency stimulation, and the rate of replenishment is low, the RRP vesicle pool is depleted more quickly than it can be replenished. As the number of vesicles in the RRP becomes rate limiting, subsequent stimuli release fewer vesicles, resulting in the reduction of PPF (Zucker and Regehr 2002). An input frequency of 20 Hz may represent the upper limit to PPF within glomerular interneurons.
Strong SAC activation blocks all MC spike output (Aungst et al. 2003; Liu et al. 2013, 2016; Whitesell et al. 2013). However, at moderate activation strengths (~50% reduction of MC spiking), inhibition of MC spiking is strongly frequency dependent. At 5–10 Hz, the test (second) of two paired pulses nearly eliminates MC spiking, whereas lower paired-pulse frequencies have no influence on MC spike output. In contrast, activation of PGCs progressively reduced MC spike output over a frequency range of 0.83–10 Hz. Thus, STP in both circuits suppresses MC spiking at physiologically frequencies, but the interglomerular network preferentially suppresses at higher frequencies.
Synaptic plasticity of glomerular inhibitory circuits (Ryan et al. 2015) may impact sensory processing (Wilson et al. 2004). Here, we show there is robust frequency-dependent, short-term facilitation of SAC and PGC inhibitory synapses onto MCs (Fig. 6). This short-term plasticity is strongest at frequencies corresponding to investigative sniffing rates (Carey and Wachowiak 2011; Verhagen et al. 2007; Wachowiak, 2011; Wesson et al. 2008). By reducing MC responses to sensory input, STP of PGC→MC synapses would predominate at lower sniff rates and enhance the impact of intraglomerular inhibition of MC firing. As sniff frequencies increase and potentiation in the SAC→MC and SAC→PGC synapses increases, inhibitory control would shift to the interglomerular network. By adjusting their sniff rates, animals may exploit this differential frequency dependence to preferentially suppress weakly activated glomeruli by interglomerular inhibition from strongly activated glomeruli and enhance transmission. Inhibition by glomerular inhibitory circuits thus may differentially tune signals transmitted to subsequent cortical networks of salient odor signals during investigative sniffing.
Fig. 6.
Diagrammatic representation of short-term plasticity between glomerular interneurons and mitral cells (MCs) in the olfactory bulb. DA, dopamine; GAD2, glutamic acid decarboxylase 2; PPF, paired-pulse facilitation; SAC, short axon cell.
GRANTS
This work was supported by National Institute of Health Grants R01DC010915 and R01DC005676.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.-W.Z., Z.-Y.S., M.T.S., and A.C.P. conceived and designed research; F.-W.Z. and Z.-Y.S. performed experiments; F.-W.Z., Z.-Y.S., M.T.S., and A.C.P. analyzed data; F.-W.Z., Z.-Y.S., M.T.S., and A.C.P. interpreted results of experiments; F.-W.Z., Z.-Y.S., and A.C.P. prepared figures; F.-W.Z., Z.-Y.S., and A.C.P. drafted manuscript; F.-W.Z., Z.-Y.S., M.T.S., and A.C.P. edited and revised manuscript; A.C.P. approved final version of manuscript.
REFERENCES
- Aroniadou-Anderjaska V, Zhou FM, Priest CA, Ennis M, Shipley MT. Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABAB heteroreceptors. J Neurophysiol 84: 1194–1203, 2000. doi: 10.1152/jn.2000.84.3.1194. [DOI] [PubMed] [Google Scholar]
- Aungst JL, Heyward PM, Puche AC, Karnup SV, Hayar A, Szabo G, Shipley MT. Centre-surround inhibition among olfactory bulb glomeruli. Nature 426: 623–629, 2003. doi: 10.1038/nature02185. [DOI] [PubMed] [Google Scholar]
- Baker H, Liu N, Chun HS, Saino S, Berlin R, Volpe B, Son JH. Phenotypic differentiation during migration of dopaminergic progenitor cells to the olfactory bulb. J Neurosci 21: 8505–8513, 2001. doi: 10.1523/JNEUROSCI.21-21-08505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Marbach F, Anselmi F, Koh MS, Davis MB, Garcia da Silva P, Delevich K, Oyibo HK, Gupta P, Li B, Albeanu DF. An interglomerular circuit gates glomerular output and implements gain control in the mouse olfactory bulb. Neuron 87: 193–207, 2015. doi: 10.1016/j.neuron.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito N, Gaborieau E, Sanz Diez A, Kosar S, Foucault L, Raineteau O, De Saint Jan D. A pool of postnatally generated interneurons persists in an immature stage in the olfactory bulb. J Neurosci 38: 9870–9882, 2018. doi: 10.1523/JNEUROSCI.1216-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisovska M, Bensen AL, Chong G, Westbrook GL. Distinct modes of dopamine and GABA release in a dual transmitter neuron. J Neurosci 33: 1790–1796, 2013. doi: 10.1523/JNEUROSCI.4342-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton SD, LaRocca G, Liu A, Cheetham CE, Urban NN. Olfactory bulb deep short-axon cells mediate widespread inhibition of tufted cell apical dendrites. J Neurosci 37: 1117–1138, 2017. doi: 10.1523/JNEUROSCI.2880-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bywalez WG, Ona-Jodar T, Lukas M, Ninkovic J, Egger V. Dendritic arborization patterns of small juxtaglomerular cell subtypes within the rodent olfactory bulb. Front Neuroanat 10: 127, 2017. doi: 10.3389/fnana.2016.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RM, Wachowiak M. Effect of sniffing on the temporal structure of mitral/tufted cell output from the olfactory bulb. J Neurosci 31: 10615–10626, 2011. doi: 10.1523/JNEUROSCI.1805-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson GC, Shipley MT, Keller A. Long-lasting depolarizations in mitral cells of the rat olfactory bulb. J Neurosci 20: 2011–2021, 2000. doi: 10.1523/JNEUROSCI.20-05-02011.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marchis S, Bovetti S, Carletti B, Hsieh YC, Garzotto D, Peretto P, Fasolo A, Puche AC, Rossi F. Generation of distinct types of periglomerular olfactory bulb interneurons during development and in adult mice: implication for intrinsic properties of the subventricular zone progenitor population. J Neurosci 27: 657–664, 2007. doi: 10.1523/JNEUROSCI.2870-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marchis S, Temoney S, Erdelyi F, Bovetti S, Bovolin P, Szabo G, Puche AC. GABAergic phenotypic differentiation of a subpopulation of subventricular derived migrating progenitors. Eur J Neurosci 20: 1307–1317, 2004. doi: 10.1111/j.1460-9568.2004.03584.x. [DOI] [PubMed] [Google Scholar]
- De Saint Jan D, Hirnet D, Westbrook GL, Charpak S. External tufted cells drive the output of olfactory bulb glomeruli. J Neurosci 29: 2043–2052, 2009. doi: 10.1523/JNEUROSCI.5317-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz D, Muñoz-Castañeda R, Ávila-Zarza C, Carretero J, Alonso JR, Weruaga E. Olfactory bulb plasticity ensures proper olfaction after severe impairment in postnatal neurogenesis. Sci Rep 7: 5654, 2017. doi: 10.1038/s41598-017-05970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English DF, McKenzie S, Evans T, Kim K, Yoon E, Buzsáki G. Pyramidal cell-interneuron circuit architecture and dynamics in hippocampal networks. Neuron 96: 505–520.e7, 2017. doi: 10.1016/j.neuron.2017.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis M, Zhou FM, Ciombor KJ, Aroniadou-Anderjaska V, Hayar A, Borrelli E, Zimmer LA, Margolis F, Shipley MT. Dopamine D2 receptor-mediated presynaptic inhibition of olfactory nerve terminals. J Neurophysiol 86: 2986–2997, 2001. doi: 10.1152/jn.2001.86.6.2986. [DOI] [PubMed] [Google Scholar]
- Fioravante D, Regehr WG. Short-term forms of presynaptic plasticity. Curr Opin Neurobiol 21: 269–274, 2011. doi: 10.1016/j.conb.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliano E, Franzoni E, Breton M, Chand AN, Byrne DJ, Murthy VN, Grubb MS. Embryonic and postnatal neurogenesis produce functionally distinct subclasses of dopaminergic neuron. eLife 7: e32373, 2018. doi: 10.7554/eLife.32373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gire DH, Franks KM, Zak JD, Tanaka KF, Whitesell JD, Mulligan AA, Hen R, Schoppa NE. Mitral cells in the olfactory bulb are mainly excited through a multistep signaling path. J Neurosci 32: 2964–2975, 2012. doi: 10.1523/JNEUROSCI.5580-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gire DH, Schoppa NE. Control of on/off glomerular signaling by a local GABAergic microcircuit in the olfactory bulb. J Neurosci 29: 13454–13464, 2009. doi: 10.1523/JNEUROSCI.2368-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey JD, Heinbockel T. Neuromodulation of synaptic transmission in the main olfactory bulb. Int J Environ Res Public Health 15: 2194, 2018. doi: 10.3390/ijerph15102194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Karnup S, Ennis M, Shipley MT. External tufted cells: a major excitatory element that coordinates glomerular activity. J Neurosci 24: 6676–6685, 2004b. doi: 10.1523/JNEUROSCI.1367-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Karnup S, Shipley MT, Ennis M. Olfactory bulb glomeruli: external tufted cells intrinsically burst at theta frequency and are entrained by patterned olfactory input. J Neurosci 24: 1190–1199, 2004a. doi: 10.1523/JNEUROSCI.4714-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Shipley MT, Ennis M. Olfactory bulb external tufted cells are synchronized by multiple intraglomerular mechanisms. J Neurosci 25: 8197–8208, 2005. doi: 10.1523/JNEUROSCI.2374-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Strowbridge BW. Olfactory reciprocal synapses: dendritic signaling in the CNS. Neuron 20: 749–761, 1998. doi: 10.1016/S0896-6273(00)81013-2. [DOI] [PubMed] [Google Scholar]
- Karpuk N, Hayar A. Activation of postsynaptic GABAB receptors modulates the bursting pattern and synaptic activity of olfactory bulb juxtaglomerular neurons. J Neurophysiol 99: 308–319, 2008. doi: 10.1152/jn.01086.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokage E, Pan YZ, Shao Z, Kobayashi K, Szabo G, Yanagawa Y, Obata K, Okano H, Toida K, Puche AC, Shipley MT. Molecular identity of periglomerular and short axon cells. J Neurosci 30: 1185–1196, 2010. doi: 10.1523/JNEUROSCI.3497-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi M, Osumi N, Rubenstein JL, Alvarez-Buylla A. Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J Neurosci 25: 6997–7003, 2005. doi: 10.1523/JNEUROSCI.1435-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka K, Toida K, Aika Y, Kosaka T. How simple is the organization of the olfactory glomerulus?: the heterogeneity of so-called periglomerular cells. Neurosci Res 30: 101–110, 1998. doi: 10.1016/S0168-0102(98)00002-9. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Kosaka K. Tyrosine hydroxylase-positive GABAergic juxtaglomerular neurons are the main source of the interglomerular connections in the mouse main olfactory bulb. Neurosci Res 60: 349–354, 2008. doi: 10.1016/j.neures.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Liu S, Plachez C, Shao Z, Puche A, Shipley MT. Olfactory bulb short axon cell release of GABA and dopamine produces a temporally biphasic inhibition-excitation response in external tufted cells. J Neurosci 33: 2916–2926, 2013. doi: 10.1523/JNEUROSCI.3607-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Puche AC, Shipley MT. The interglomerular circuit potently inhibits olfactory bulb output neurons by both direct and indirect pathways. J Neurosci 36: 9604–9617, 2016. doi: 10.1523/JNEUROSCI.1763-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Shipley MT. Multiple conductances cooperatively regulate spontaneous bursting in mouse olfactory bulb external tufted cells. J Neurosci 28: 1625–1639, 2008. doi: 10.1523/JNEUROSCI.3906-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science 264: 1145–1148, 1994. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron 11: 173–189, 1993. doi: 10.1016/0896-6273(93)90281-U. [DOI] [PubMed] [Google Scholar]
- Mainland J, Sobel N. The sniff is part of the olfactory percept. Chem Senses 31: 181–196, 2006. doi: 10.1093/chemse/bjj012. [DOI] [PubMed] [Google Scholar]
- Matveev V, Wang XJ. Differential short-term synaptic plasticity and transmission of complex spike trains: to depress or to facilitate? Cereb Cortex 10: 1143–1153, 2000. doi: 10.1093/cercor/10.11.1143. [DOI] [PubMed] [Google Scholar]
- McGann JP, Pírez N, Gainey MA, Muratore C, Elias AS, Wachowiak M. Odorant representations are modulated by intra- but not interglomerular presynaptic inhibition of olfactory sensory neurons. Neuron 48: 1039–1053, 2005. doi: 10.1016/j.neuron.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Glickfeld LL, Balsen Z, Isaacson JS. Sensory neuron signaling to the brain: properties of transmitter release from olfactory nerve terminals. J Neurosci 24: 3023–3030, 2004. doi: 10.1523/JNEUROSCI.5745-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama S, Homma R, Imamura F. Neuronal organization of olfactory bulb circuits. Front Neural Circuits 8: 98, 2014. doi: 10.3389/fncir.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najac M, De Saint Jan D, Reguero L, Grandes P, Charpak S. Monosynaptic and polysynaptic feed-forward inputs to mitral cells from olfactory sensory neurons. J Neurosci 31: 8722–8729, 2011. doi: 10.1523/JNEUROSCI.0527-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najac M, Sanz Diez A, Kumar A, Benito N, Charpak S, De Saint Jan D. Intraglomerular lateral inhibition promotes spike timing variability in principal neurons of the olfactory bulb. J Neurosci 35: 4319–4331, 2015. doi: 10.1523/JNEUROSCI.2181-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish-Aungst S, Shipley MT, Erdelyi F, Szabo G, Puche AC. Quantitative analysis of neuronal diversity in the mouse olfactory bulb. J Comp Neurol 501: 825–836, 2007. doi: 10.1002/cne.21205. [DOI] [PubMed] [Google Scholar]
- Parsa PV, D’Souza RD, Vijayaraghavan S. Signaling between periglomerular cells reveals a bimodal role for GABA in modulating glomerular microcircuitry in the olfactory bulb. Proc Natl Acad Sci USA 112: 9478–9483, 2015. doi: 10.1073/pnas.1424406112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Lim JH, Brunjes PC. Activity-dependent regulation of calcium-binding proteins in the developing rat olfactory bulb. J Comp Neurol 387: 12–26, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- Plachez C, Puche AC. Early specification of GAD67 subventricular derived olfactory interneurons. J Mol Histol 43: 215–221, 2012. doi: 10.1007/s10735-012-9394-2. [DOI] [PubMed] [Google Scholar]
- Price JL, Powell TP. The mitral and short axon cells of the olfactory bulb. J Cell Sci 7: 631–651, 1970. [DOI] [PubMed] [Google Scholar]
- Ryan TJ, Roy DS, Pignatelli M, Arons A, Tonegawa S. Engram cells retain memory under retrograde amnesia. Science 348: 1007–1013, 2015. doi: 10.1126/science.aaa5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Liu S, Zhou F, Puche AC, Shipley MT. Reciprocal inhibitory glomerular circuits contribute to excitation-inhibition balance in the mouse olfactory bulb. eNeuro 6: ENEURO.0048-19.2019, 2019. doi: 10.1523/ENEURO.0048-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Puche AC, Kiyokage E, Szabo G, Shipley MT. Two GABAergic intraglomerular circuits differentially regulate tonic and phasic presynaptic inhibition of olfactory nerve terminals. J Neurophysiol 101: 1988–2001, 2009. doi: 10.1152/jn.91116.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Puche AC, Liu S, Shipley MT. Intraglomerular inhibition shapes the strength and temporal structure of glomerular output. J Neurophysiol 108: 782–793, 2012. doi: 10.1152/jn.00119.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Puche AC, Shipley MT. Intraglomerular inhibition maintains mitral cell response contrast across input frequencies. J Neurophysiol 110: 2185–2191, 2013. doi: 10.1152/jn.00023.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley CH, Coddington EJ, Heyward PM. All-or-none population bursts temporally constrain surround inhibition between mouse olfactory glomeruli. Brain Res Bull 81: 406–415, 2010. doi: 10.1016/j.brainresbull.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Sirotin YB, Costa ME, Laplagne DA. Rodent ultrasonic vocalizations are bound to active sniffing behavior. Front Behav Neurosci 8: 399, 2014. doi: 10.3389/fnbeh.2014.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanawala MS, Regehr WG. Determining synaptic parameters using high-frequency activation. J Neurosci Methods 264: 136–152, 2016. doi: 10.1016/j.jneumeth.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toida K, Kosaka K, Heizmann CW, Kosaka T. Synaptic contacts between mitral/tufted cells and GABAergic neurons containing calcium-binding protein parvalbumin in the rat olfactory bulb, with special reference to reciprocal synapses between them. Brain Res 650: 347–352, 1994. doi: 10.1016/0006-8993(94)91804-X. [DOI] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324: 1080–1084, 2009. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Mainen ZF. Speed and accuracy of olfactory discrimination in the rat. Nat Neurosci 6: 1224–1229, 2003. doi: 10.1038/nn1142. [DOI] [PubMed] [Google Scholar]
- Vaaga CE, Yorgason JT, Williams JT, Westbrook GL. Presynaptic gain control by endogenous cotransmission of dopamine and GABA in the olfactory bulb. J Neurophysiol 117: 1163–1170, 2017. doi: 10.1152/jn.00694.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen JV, Wesson DW, Netoff TI, White JA, Wachowiak M. Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nat Neurosci 10: 631–639, 2007. doi: 10.1038/nn1892. [DOI] [PubMed] [Google Scholar]
- Vučinić D, Cohen LB, Kosmidis EK. Interglomerular center-surround inhibition shapes odorant-evoked input to the mouse olfactory bulb in vivo. J Neurophysiol 95: 1881–1887, 2006. doi: 10.1152/jn.00918.2005. [DOI] [PubMed] [Google Scholar]
- Wachowiak M. All in a sniff: olfaction as a model for active sensing. Neuron 71: 962–973, 2011. doi: 10.1016/j.neuron.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachowiak M, Cohen LB. Presynaptic inhibition of primary olfactory afferents mediated by different mechanisms in lobster and turtle. J Neurosci 19: 8808–8817, 1999. doi: 10.1523/JNEUROSCI.19-20-08808.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachowiak M, Shipley MT. Coding and synaptic processing of sensory information in the glomerular layer of the olfactory bulb. Semin Cell Dev Biol 17: 411–423, 2006. doi: 10.1016/j.semcdb.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Wesson DW, Donahou TN, Johnson MO, Wachowiak M. Sniffing behavior of mice during performance in odor-guided tasks. Chem Senses 33: 581–596, 2008. doi: 10.1093/chemse/bjn029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesell JD, Sorensen KA, Jarvie BC, Hentges ST, Schoppa NE. Interglomerular lateral inhibition targeted on external tufted cells in the olfactory bulb. J Neurosci 33: 1552–1563, 2013. doi: 10.1523/JNEUROSCI.3410-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA, Best AR, Sullivan RM. Plasticity in the olfactory system: lessons for the neurobiology of memory. Neuroscientist 10: 513–524, 2004. doi: 10.1177/1073858404267048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FW, Dong HW, Ennis M. Activation of β-noradrenergic receptors enhances rhythmic bursting in mouse olfactory bulb external tufted cells. J Neurophysiol 116: 2604–2614, 2016. doi: 10.1152/jn.00034.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FW, Matta SG, Zhou FM. Constitutively active TRPC3 channels regulate basal ganglia output neurons. J Neurosci 28: 473–482, 2008. doi: 10.1523/JNEUROSCI.3978-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FW, Puche AC, Shipley MT. Short-term plasticity at olfactory cortex to granule cell synapses requires CaV2.1 activation. Front Cell Neurosci 12: 387, 2018. doi: 10.3389/fncel.2018.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FW, Roper SN. Altered firing rates and patterns in interneurons in experimental cortical dysplasia. Cereb Cortex 21: 1645–1658, 2011. doi: 10.1093/cercor/bhq234. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol 64: 355–405, 2002. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]