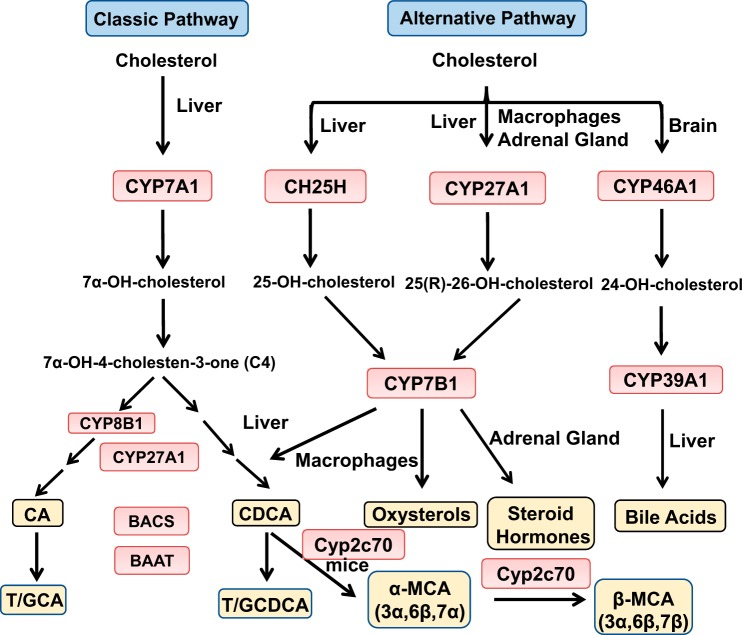
Fig. 1.
Bile acid synthesis in the liver. The classic bile acid synthesis pathway is initiated by cholesterol 7α-hydroxylase (CYP7A1). Sterol 12α-hydroxylase (CYP8B1) is involved in synthesis of cholic acid (CA), and mitochondrial sterol 27-hydroxylase (CYP27A1) catalyzes steroid side-chain oxidation. CA and chenodeoxycholic acid (CDCA) are the two major primary bile acids synthesized in the human liver. Bile acid-CoA synthase (BACS) catalyzes the addition of a CoA group and then bile acid-CoA-to-amino acid transferase (BAAT) adds taurine (T) or glycine (G) to form T/G-conjugated bile acids for secretion in bile. The alternative bile acid synthesis pathway is initiated by hydroxylation of the steroid side chain, followed by steroid ring modifications. In the liver, cholesterol is hydroxylated by sterol 25-hydroxylase and CYP27A1 to form 25-hydroxycholesterol and 27-hydroxycholesterol [also named 25(R)-26-hydroxycholesterol], respectively, which are then hydroxylated at the 7α-position by oxysterol 7α-hydroxylase (CYP7B1). CYP27A1 and CYP7B1 are widely expressed in macrophages, adrenal glands, and other tissues. In the brain, sterol 24-hydroxylase (CYP46A1) converts cholesterol to 24-hydroxycholesterol, which is 7α-hydroxylated by sterol 7α-hydroxylase (CYP39A1) in the liver. In mice, CDCA is converted to α-muricholic acid (MCA) and β-MCA by Cyp2c70.