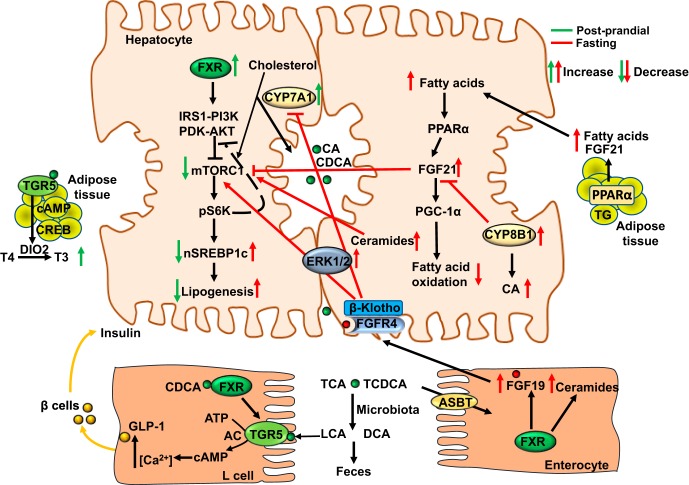
Fig. 5.
Nutrient regulation of bile acid synthesis and hepatic metabolism. Feeding rapidly induces cholesterol 7α-hydroxylase (CYP7A1) but inhibits sterol 12α-hydroxylase (CYP8B1) expression to increase bile acid synthesis and aid in nutrient absorption during the postprandial state. Stimulating bile acid synthesis induces hepatic autophagy via insulin/AKT signaling to inhibit the mammalian target of rapamycin complex 1 (mTORC1). Farnesoid X receptor (FXR) activates insulin receptor substrate 1-AKT (protein kinase B) signaling to inhibit mTORC1/pS6K-signaling to promote nuclear translocation of sterol regulatory element binding protein 1c (nSREBP-1c) and lipogenesis. During the postprandial state, glucose is transported to enterocytes via the sodium-glucose-cotransporter 2. Chenodeoxycholic acid (CDCA) activates FXR in enteroendocrine L cells to induce Takeda G protein-coupled receptor 5 (TGR5) signaling and stimulate glucose-induced glucagon-like peptide-1 (GLP-1) secretion via increased intracellular cAMP and Ca2+. GLP-1 promotes insulin secretion from pancreatic β cells and increases insulin sensitivity. Activation of TGR5 in brown adipose tissue stimulates energy metabolism and the conversion of thyroxine to 3,5,3′-triiodothyronine. In the late post-prandial state to postabsorptive state, intestinal FXR induces FGF19, which activates hepatic FGF receptor (FGFR) 4-ERK1/2 signaling to inhibit bile acid synthesis. FGF19 regulates glucose metabolism, glycogen synthesis, and protein synthesis when insulin levels are decreased. In the intestine, activation of FXR induces ceramide synthesis. Ceramides activate mTORC1 signaling to induce SREBP-1c-mediated lipogenesis and induce ER stress and ROS to cause insulin resistance. During prolonged fasting or starvation, CYP7A1 expression and bile acid synthesis is reduced, but CYP8B1 is induced to increase chloric acid (CA) synthesis and deoxycholic acid (DCA) content in the colon, promoting ceramide synthesis and SREBP-1c-mediated lipogenesis. Increasing free fatty acids activate peroxisome proliferator-activated receptor (PPAR) α and PPARγ coactivator protein-1α (PGC-1α) to stimulate FGF21 production in liver and adipose tissue. As an adaptation to prolonged fasting to maintain metabolic homeostasis, increased FGF21 stimulates glucose and energy metabolism independent of insulin. FGF21 reduces serum triglycerides by stimulating energy metabolism in adipose tissue. FGF21 also inhibits mTORC1 signaling to improve insulin sensitivity and reduce hepatic steatosis. Green arrows indicate changes in the postprandial state and red arrows indicate changes in the fasting state. ABST, apical sodium-dependent bile acid transporter AC, adenylyl cyclase; CREB, cAMP response element binding protein; DIO2, deiodinase type 2; IRS1, insulin-resistant substrate 1; LCA, lithocholic acid; PDK, phosphoinositide-dependent kinase; PI3K, phosphoinositide 3-kinase; TG, triglycerides.