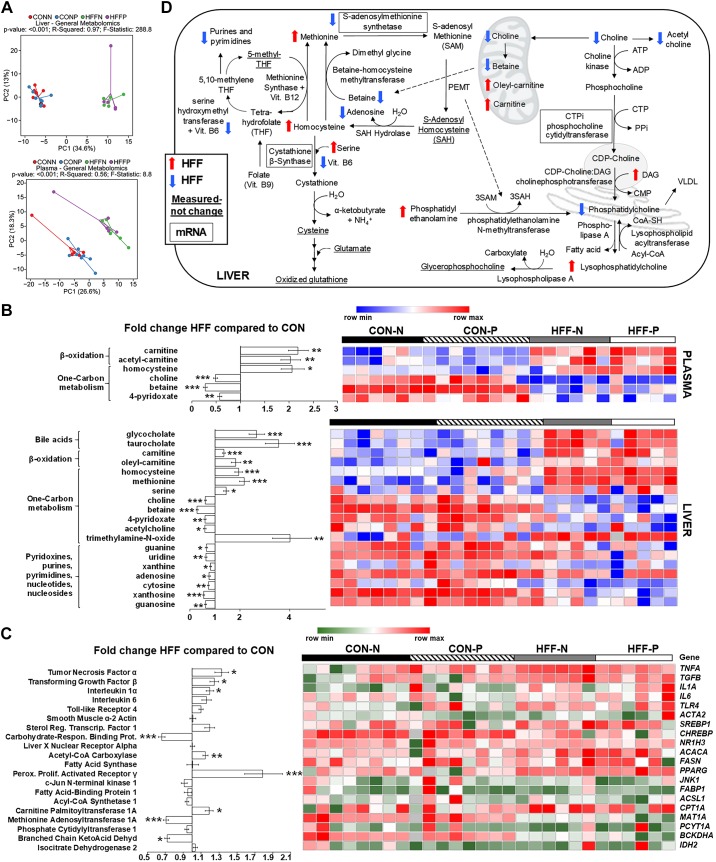
Fig. 3.
Metabolic changes in high-fructose, high-fat (HFF) livers and plasma were consistent with dysregulation of one-carbon metabolism. A: principal component analysis [principal components 1 and 2 (PC1 and PC2)] to visualize group discrimination in a two-dimensional scores plot showed a separation of HFF and control (CON) samples, but not a probiotic effect. Each point represents an individual pig, and color of point denotes diet. Group differences were assessed by nonparametric permutational analysis of variance with diet × probiotic as fixed effects and pen nested in diet × probiotic as random effect, under a reduced model, 9,999 permutations, and type III sum of squares. N, nonprobiotic; P, probiotic. B: heat map of absolute abundance of metabolites significantly altered by the diet in plasma and liver samples of 83-day-old pigs, with fold change and significance levels by HFF compared with CON. Only metabolites related to bile acids, β-oxidation, one-carbon metabolism, and nucleic acids are shown. Columns are individual pigs, and rows are log2-transformed metabolites. Blue and red represent the row minimum and maximum values, respectively. C: heat map of relative mRNA abundance of genes related to inflammation, one-carbon metabolism, de novo lipogenesis, and fat transport and β-oxidation in the liver, measured by qPCR in 83-day-old pigs. Columns are individual pigs, and rows are ΔCt values. Green and red represent the row minimum and maximum values, respectively. Fold change was calculated as 2−ΔΔCt. D: mechanistic illustration of pathways involved in hepatic one-carbon and lipid metabolism, and relevant metabolites and genes altered by the diet. Blue and red arrows represent increased and decreased expression in HFF-fed pigs compared with CON, respectively. A decrease in choline availability may have reduced the hepatic synthesis of phosphatidylcholines (PCs) via the cytidine 5′-diphosphocholine pathway, decreasing the production of lipoproteins and export of fat and cholesterol from the liver. The increase in phosphatidylethanolamines (PEs), lysophosphatidylethanolamines (LPEs), and lysophosphatidylcholines (LPCs) in HFF livers suggests also an impairment in the hepatic synthesis of PCs via phosphatidylethanolamine N-methyltransferase (PEMT) and lysophospholipid-acyltransferase pathways. Because PEMT requires three S-adenosyl methionine (SAM) molecules to convert a PE into a PC, it is possible that HFF-fed pigs became deficient in SAM. In this regard, HFF livers had increased homocysteine and methionine levels, and a decrease in SAM synthetase expression, which catalyzes the conversion of methionine into SAM. Furthermore, 4-pyridoxate, purines and pyrimidines were lower in HFF, indicative of a deficit in folate cycle intermediates, which may have impaired the remethylation of homocysteine via the methionine synthase pathway, while a decrease in betaine may have reduced substrate availability for betaine-homocysteine methyl transferase. P values were calculated by a two-way ANOVA with a mixed model and adjusted for multiple testing with Benjamin-Hochberg procedure and Tukey’s post hoc test. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.