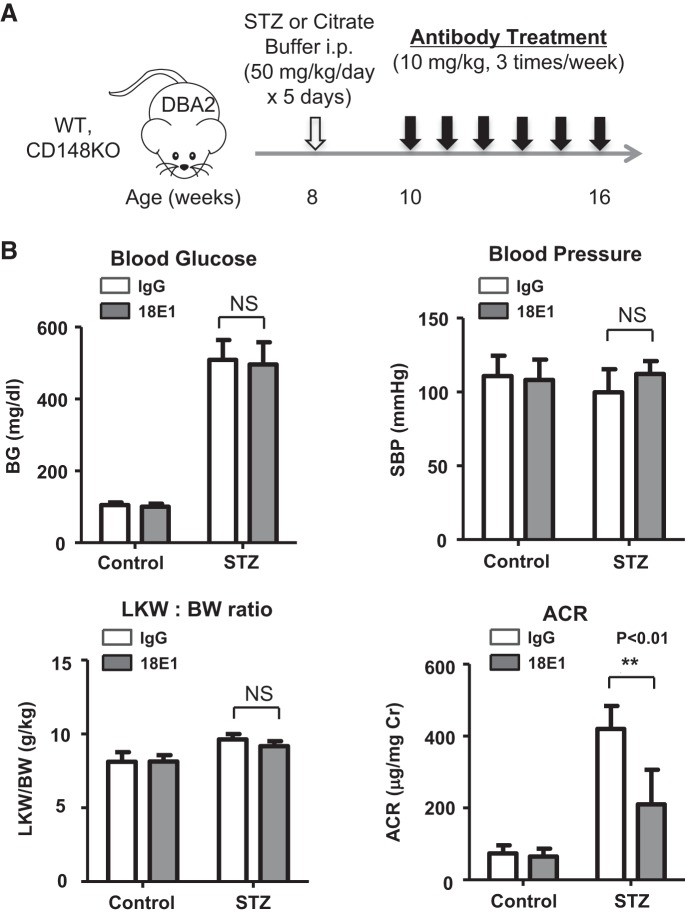
Fig. 3.
Effects of 18E1 monoclonal antibody (mAb) in murine diabetic nephropathy (DN). A: experimental protocol of the 18E1 mAb treatment study. Diabetes was induced in male mice of the DBA/2J strain at the age of 8 wk by multiple low-dose streptozotocin (STZ) injections. Mice injected with citrate buffer were used as nondiabetic controls. 18E1 mAb or control IgG was injected (10 mg/kg ip, 3 times/wk) in diabetic (STZ) or nondiabetic (control) mice for 6 wk, starting 2 wk after STZ injections. Measurements and tissue sampling were performed before (data not shown) and 6 wk after antibody treatment (8 wk after STZ or citrate buffer injections). B: effects of 6 wk 18E1 mAb or control IgG treatment on blood glucose (BG), systolic blood pressure (SBP), left kidney weight-to-body weight ratio (LKW/BW), and urinary albumin-to-creatinine ratio (ACR) were assessed in diabetic (STZ) and nondiabetic (control) mice as described in materials and methods. n = 8 mice/group. Data are presented as means ± SE. **P < 0.01 vs. control IgG-treated mice.