Fig. 7.4.
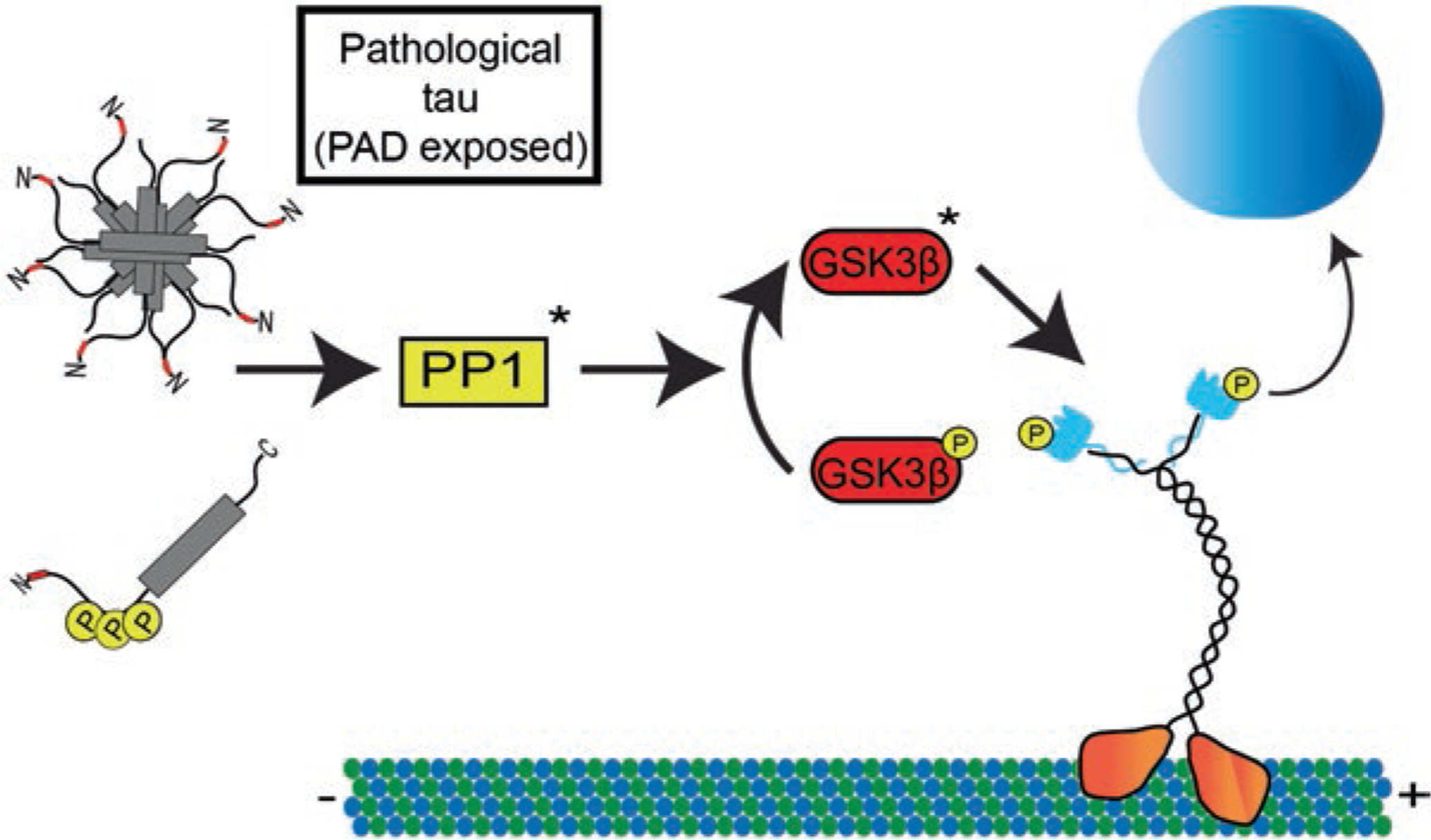
Tau alters kinesin-cargo interactions through modulation of a PP1-GSK3β signaling pathway. Tau contains a phosphatase-activating domain (PAD) within amino acids 2–18 at the extreme N-terminus of tau (shown in red). Under normal conditions this epitope is obscured allowing for normal kinesin-based transport along the microtubules. In disease conditions tau can undergo a variety of modifications including aggregation or specific phosphorylation events that can aberrantly expose the PAD epitope. In the proposed model, these forms of tau can disrupt normal kinesin-based transport by activating protein phosphatase 1 (PP1). This in turn dephosphorylates an inhibitory phosphate on GSK3β in order to activate it. GSK3β then phosphorylates kinesin light chain inducing a release of cargo and disruption of FAT
