Figure 2.
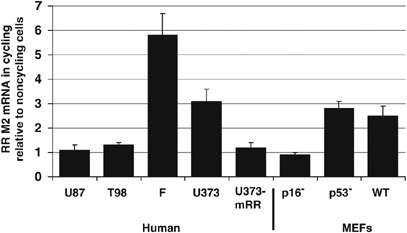
Ribonucleotide reductase expression is elevated in noncycling cells with p16 deletions. Reverse transcription (RT)–PCR shows the ratio of M2 subunit of mammalian ribonucleotide reductase (mRR) mRNA in cycling relative to noncycling cells was 0.9–1.3 in p16-deleted cells (U87, T98, and p16−/− MEFs). However, in p16-expressing cells (that is, wild-type MEFs, human fibroblasts, p53−/− MEFs and U373), this ratio was much higher (2.5–5.8). A high capacity cDNA archive kit (Applied Biosystems, Foster City, CA, USA) was used to generate cDNA from RNA isolated from cells using Trizol (Invitrogen) per protocol. Real-time RT–PCR was performed on an ABI Prism 7000 (Applied Biosystems) machine using human primer-probe combinations for RR subunit M2 (Applied Biosystems part nos. Hs00357247_g1 and Hs00168784_m1) and 18S rRNA (Applied Biosystems part no. 4308329) combined with TaqMan Master Mix (Applied Biosystems). Relative quantification was performed using 18S rRNA as an endogenous control. All reactions began with 10 min at 95 °C for AmpliTaq Gold activation, followed by 40 cycles at 95 °C for 15 s for denaturation and then 60 °C for 1 min for annealing/extension. Abbreviations used: F, fibroblasts; WT, wild-type; U373-mRR, U373 clone selected after stable transfection with plasmid constitutively expressing the M2 subunit of mammalian ribonucleotide reductase.
