Fig. 4.
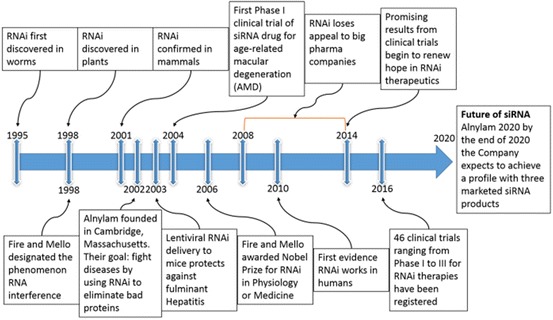
RNAi discovery timeline. 1995: First indication that sense RNA was as effective as antisense RNA for suppressing gene expression in C. elegans [26]. 1998: Waterhouse describes the process of RNAi in plants, whilst Fire and Mello coin the term “RNA interference” [12, 24]. 2001: First evidence that the RNAi mechanism is present in mammals [27]. 2002: Alnylam Pharmaceuticals founded by Phillip D. Zamore, Thomas Tuschl and Phillip Sharp. 2003: First evidence that RNAi is effective in inhibiting fulminant hepatitis virus in a mouse model [28]. 2004: Acuity Pharmaceuticals performs first phase I clinical trial of siRNA. 2006: Andrew Fire and Craig Mello awarded the Nobel Prize in Physiology or Medicine for RNA interference–gene silencing by double-stranded RNA. 2008–2014: Due to lack of success in clinical trials, many of the large pharmaceutical companies stop investing in siRNA technology. 2010: Davies describes first evidence of siRNA-mediated inhibition in humans [29]. 2014: RNAi begins to show promising clinical progress [30]. 2016: A total of 46 registered siRNA clinical trials for RNAi therapeutics from phase I to phase III trials, most involving siRNA NP formulations (http://clinicaltrials.gov). Future: By the end of 2020, Alnylam Pharmaceuticals, still the largest RNAi-dedicated company, expects to achieve a company profile with three marketed products and ten RNAi therapeutic clinical programs—including four in late stages of development (http://www.alnylam.com)
