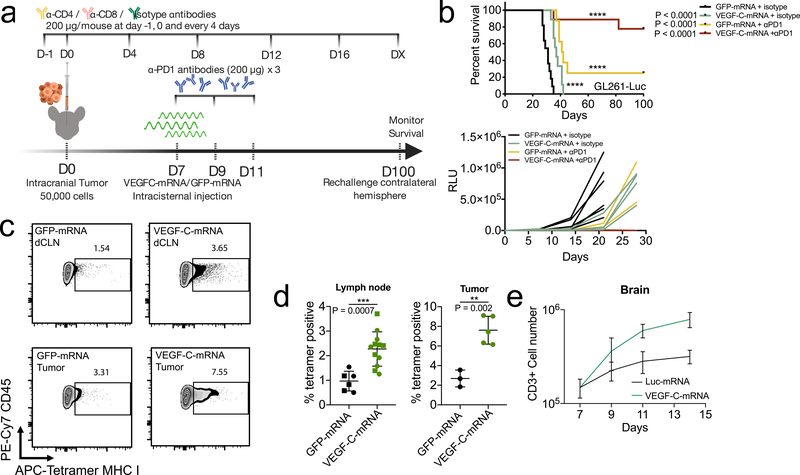
Figure 3. Therapeutic delivery of VEGF-C potentiates checkpoint inhibitor therapy by increased T cell priming.
a Schematic of treatment plans for (b). b Mice inoculated with 50,000 GL261-Luc cells were treated with VEGF-C-mRNA/GFP-mRNA (day 7) with either anti-PD1 (RMP1–14) antibodies or isotype antibodies (day 7, 9 and 11) and monitored for survival (kaplan meier curve; GFP-mRNA + isotype, n = 11; VEGF-C-mRNA + isotype, n = 9; GFP-mRNA + αPD1, n = 8; VEGF-C-mRNA + αPD1, n = 10, Data are pooled from 2 independent experiments) (tumor burden measurement; GFP-mRNA + isotype, n = 7; VEGF-C-mRNA + isotype, n = 5; GFP-mRNA + αPD1, n = 4; VEGF-C-mRNA + αPD1, n = 5). c-d Mice were inoculated with 50,000 GL261-Luc cells and treated with GFP-mRNA or VEGF-C-mRNA at day 7. Seven days after mRNA treatment, dcLNs and tumor bearing-brain hemisphere were collected to detect tetramer positive CD8 T cells. c Concatenated FACS plot of CD45+CD3+CD8+CD44+ T cells in tumor-bearing brain and dcLNs with GFP-mRNA or VEGF-C-mRNA treatment. Percent quantification of dcLNs (d, circle, ipsilateral; square, contralateral) or tumor infiltrating-tetramer positive CD8 T cells (Lymph node; GFP-mRNA n = 6; VEGF-C-mRNA, n = 12) (Tumor; GFP-mRNA n = 3; VEGF-C-mRNA, n = 5). Experiment was repeated independently with similar results. e Mice were inoculated with 50,000 GL261-Luc cells and treated with Luc-mRNA or VEGF-C-mRNA at day 7. Tumor inoculated brain hemisphere was collected and analyzed using FACS (n = 3; 3 animals were pooled for each n). e Number of CD3-positive cells (n = 3; 3 animals were pooled for each n). Data are mean ± S.D. *P < 0.05; **P < 0.01; ***P <0.001; ****P<0.0001 (two-tailed unpaired Student’s t-test, two-sided Log-rank Mantel-Cox test).