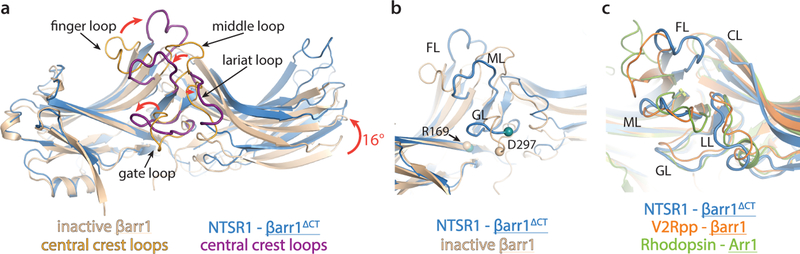
Figure 3 – NTSR1-bound βarr1ΔCT shows activation hallmarks, with some loops in distinct conformations.
a) Overlay of NTSR1-bound βarr1ΔCT (blue) with inactive βarr1 (wheat). NTSR1-bound βarr1ΔCT undergoes a 16° interdomain twist, a feature observed to varying degrees in all active-state arrestin structures (Fig S8a) and substantial rearrangements of the central crest loops (highlighted in purple and orange, respectively). b) The position of residue D297 (shown as Cα sphere) in the gate loop of the NTSR1-βarr1ΔCT complex is too far away from R169 (shown as Cα sphere) to stabilize the polar core in the inactive state. c) Compared to the active state Rho-bound Arr1 (green) and V2Rpp-bound βarr1 (orange), the middle loop (ML), gate loop (GL) and lariat loop (LL) adopt similar conformations, while the finger loop (FL) and the C-loop (CL) adopt more distinct conformations, likely due to the unique receptor-arrestin orientation we observe.