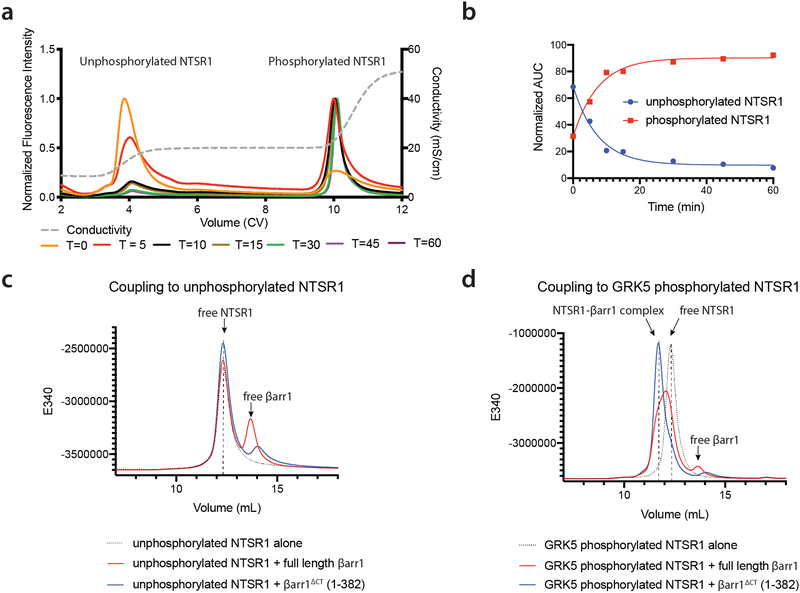
Extended Data Figure 2 – Phosphorylation of NTSR1 is crucial for βarr1 coupling.
a) The phosphorylation state of NTSR1 was assessed using ion-exchange chromatography (IEX). Overlaid chromatograms of aliquots taken at various time point (shown in minutes), the relative amounts of unphosphorylated and phosphorylated species were measured using an optimized stepped elution profile. This experiment was performed independently once. b) Graphical representation of normalized relative ratio of phosphorylated and unphosphorylated receptor from data shown in (a). FSEC chromatogram for screening βarr1 constructs for forming stable complex with either unphosphorylated (c), or GRK5-phosphorylated NTSR1 (d). Elution volumes for free receptor, free βarr1 and receptor-βarr1 complex are noted. Unphosphorylated receptor does not complex to any of the βarr1 constructs tested, while phosphorylated receptor couples moderately to full-length βarr1 and strongly to the pre-activated βarr1ΔCT construct. Experiments were performed independently twice, with similar results.