Figure 2.
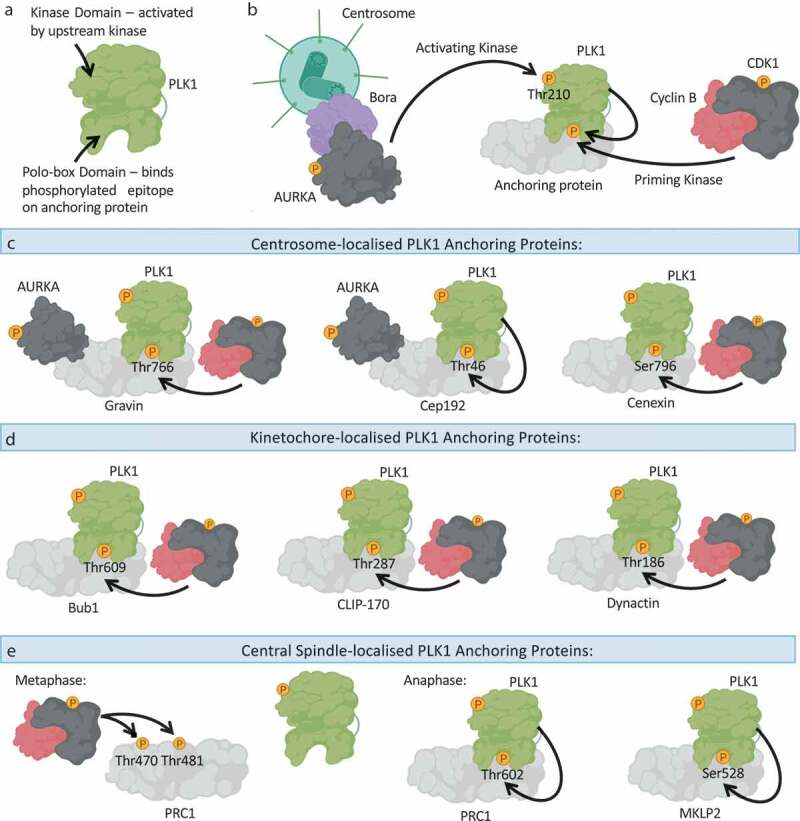
The polo-box domain determines the subcellular distribution of PLK1.
(a): Schematic overview of PLK1 domain architecture, highlighting the N-terminal kinase domain, which needs to be activated by an upstream kinase, and the C-terminal Polo-box Domain (PBD), which binds to phosphorylated epitopes on anchoring proteins to promote PLK1 association with the anchoring protein. (b): PLK1 is activated through phosphorylation of Thr210 by an upstream kinase, principally thought to be the AURKA-Bora complex. The PBD of PLK1 determines the localization of PLK1 and its recruitment to substrates, by binding to phosphorylated anchoring proteins. Such phosphorylated anchoring proteins are phosphorylated by a priming kinase, most frequently CDK1-Cyclin B or PLK1 itself. (c): An overview of the centrosome-localized PLK1 anchoring proteins: Gravin and Cep192 simultaneously bind PLK1 and AURKA. Whilst Gravin interacts with PLK1 following priming phosphorylation by CDK1, the Cep192-PLK1 interaction is thought to rely on PLK1-dependent phosphorylation of Cep192. Cenexin is another example of a centrosome-localized CDK1-dependent PLK1 anchoring protein. (d): An overview of kinetochore-localized PLK1 anchoring proteins: Bub1, CLIP-170 and Dynactin promote PLK1-recruitment to kinetochores in a CDK1-dependent manner. (e): An overview of central spindle-localized PLK1 anchoring proteins. During metaphase CDK1 activity is high, and CDK1-Cyclin B phosphorylates PRC1 on Thr470 and Thr481 to inhibit the association of PRC1 with PLK1. During anaphase, CDK1 activity is reduced, and PLK1 can phosphorylate PRC1 on Thr602 to promote the PRC1-PLK1 interaction, and concurrent recruitment of PLK1 to the central spindle. MKLP2 is another central spindle protein that anchors PLK1 to the central spindle, following PLK1-dependent phosphorylation of MKLP2 on Ser528. Created with BioRender.com.
