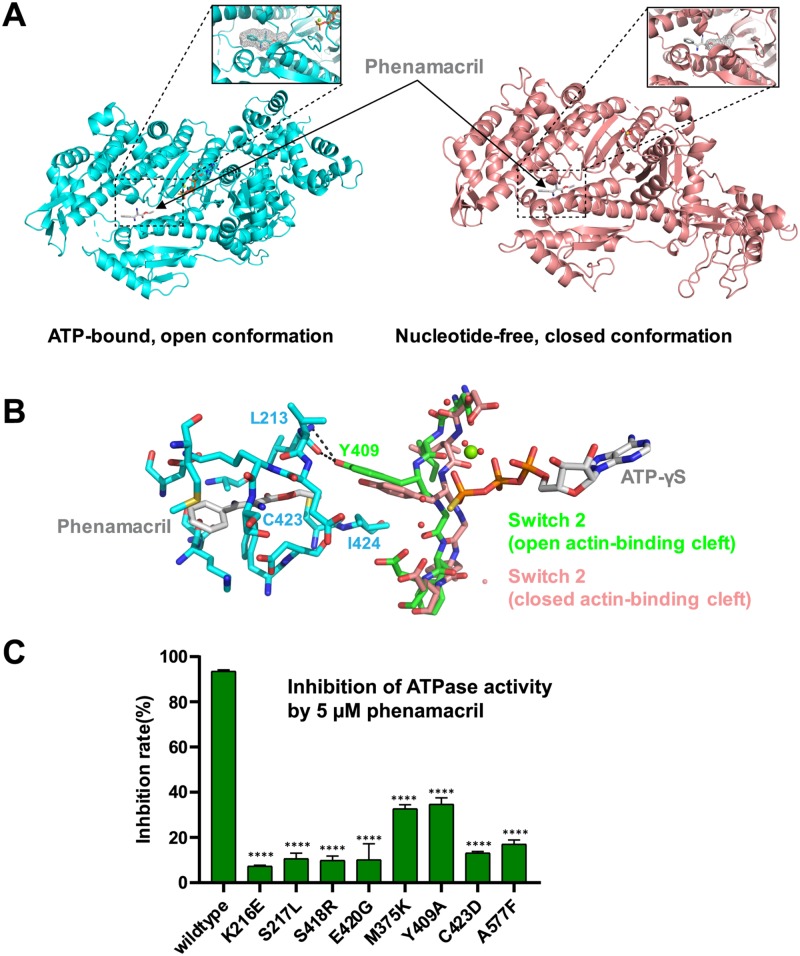
Fig 5. Phenamacril blocks closure of the actin-binding cleft.
(A) Side-by-side structural alignment of the myosin motor domain in closed (ATP-γS/phenamacril-bound FgMyoI) and open (nucleotide-free chicken myosin V) conformation overlaid with the position of phenamacril from the FgMyoI structure. The inserts show the phenamacril pocket as mesh in the open conformation and the corresponding pocket in the closed conformation. (B) Switch 2 Y409 binds the phenamacril pocket. Fg Switch 2 in the open (pre-powerstroke) conformation is shown in green, the Switch 2 conformation of chicken myosin V in closed conformation is shown in pink. (C) Phenamacril pocket mutations relieve inhibition of FgMyoI ATPase activity. Reactions contained 0.5 μM myosin and 0.1 μM calmodulin. n = 3, error bars = SD. ****: p-value<0.0001 (One-way ANOVA).