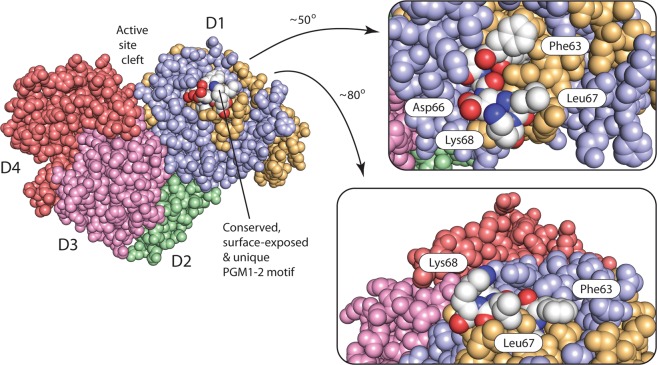
Figure 4.
PGM1-2 has a unique, surface-exposed, highly conserved patch of residues. A conserved FFSIDLK motif (residues 62 to 68, with carbons, nitrogens, and oxygens, as white, dark blue, and dark red, respectively) is unique to PGM1-2. Surface exposed Phe63, Asp66, Leu67, and Lys68 (only conserved as positively charged) are completely conserved in vertebrate PGM1-2 and are ideally located to interact with a PGM1-2 specific binding partner. The PGM1-2 protein is shown as a space-filling CPK calotte model with identical coloring as in Fig. 2a. Domains D4, D3, and D2 are shown in red, pink, and green, respectively. The PGM1-2 isoform specific exon 1-2 encoded segment is shown in light orange/white, while the rest of D1 is colored blue. Parts of the PGM1-2 structure (left) are shown magnified and rotated roughly 50° (top, right) and 80° (bottom, right) around two different axes.