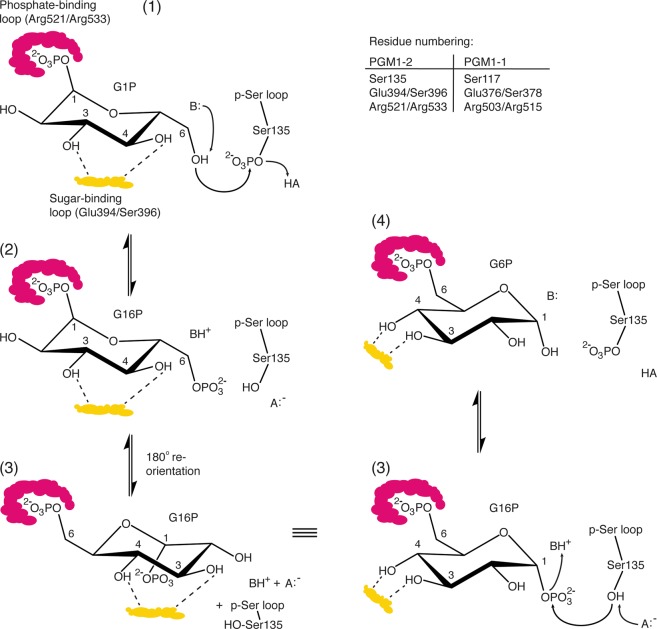
Figure 6.
Detailed PGM1 catalytic mechanism for the reversible conversion of G1P to G6P. In the substrate complex (1), the phosphate-binding loop (red) is anchoring the G1P phosphoryl group, while equatorially located hydroxyls at C3 and C4 are recognized by the sugar-binding loop (yellow). The hydroxyl group at C6 is ideally located for attack on the p-Ser phosphoryl group. After phosphoryl-transfer (2) the bisphosphorylated glucose (G16P) is poised for a 180° reorientation (“flip”) in the active site, most likely accompanied with an opening of the active site due to the flexibility of D4. Upon reorientation, G16P is again interacting with the sugar-binding loop through C3 and C4 hydroxyls, but these groups are now interchanged (3). Finally, the phosphoryl group is transferred back to the active site Ser residue on D1 (4), reactivating the PGM1 enzyme.