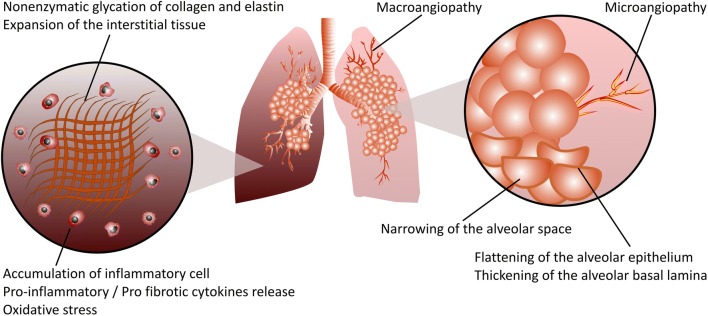
Fig. 4.
Schematic representation of DM-induced pulmonary fibrosis. Hyperglycemia, the main feature of both T1DM and T2DM, increases the accumulation of advanced glycation end product (AGE)-modified proteins, resulting in lung structural remodeling in pulmonary fibrosis. Excessive production of extracellular matrix (ECM) components such as collagen and elastin, and particularly, nonenzymatic protein glycation of the ECM due to hyperglycemia, results in matrix stiffening, which irreversibly remodels the lung tissue structure and promotes the progression of pulmonary fibrosis. Micro- and macroangiopathy, narrowing of the alveolar space, flattening of the alveolar epithelium and thickening of the alveolar basal lamina are among the structural modifications in diabetic lung fibrosis. Infiltration of both innate and adaptive immune cells into the lung tissues of diabetic individuals leads to a profound release of numerous proinflammatory/profibrotic cytokines, resulting in pulmonary fibrotic responses. Reactive oxygen species (ROS) such as superoxide and reactive nitrogen species such as peroxynitrite cause cellular and subcellular structural damage within the lung, accelerating the progression of pulmonary fibrosis