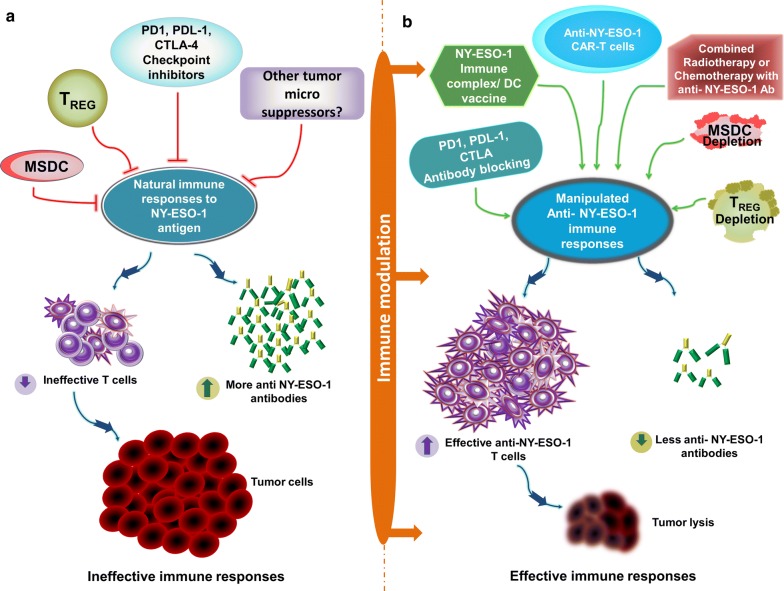
Fig. 2.
Cancer Immunotherapeutic strategies targeting NY-ESO-1 antigen. a NY-ESO-1 exhibits the capacity to induce a strong natural anti-NY-ESO-1 antibody, CD4 + and CD8 + T cell responses in an integrated manner. Effective tumor control against NY-ESO-1 expressing tumors is compromised due to strong interplay of the immune checkpoint inhibitory molecules such as programmed death 1 [PD-1], programmed death-ligand 1 [PDL-1], cytotoxic T-lymphocyte-associated protein 4 [CTLA-4] and other immune-suppressive tumor microenvironment cells such as regulatory T cells (TREG) and myeloid-derived suppressor cells (MDSC). In the presence of these immune inhibitory and immune suppressive cells, high titers of anti-NY-ESO-1 antibodies are observed while the anti-NY-ESO-1 T cell responses become ineffective. This leads to limited objective clinical responses to control tumors. b To strengthen the induction of effective anti-NY-ESO-1 specific CD4 + and CD8 + T cell immune responses and to reverse immunosuppression, various immune-modulation strategies including anti-PD-1, anti-PDL-1, anti-CTLA-4 blocking antibodies, TREG and MDSC depletion, NY-ESO-1 immune complex/dendritic cells (DC) vaccine, anti-NY-ESO-1 chimeric antigen receptor T cells (CAR T), either alone or in combination with standard of care therapies such as radiotherapy and chemotherapy can be designed for enhanced anti-NY-ESO-1 T cells responses leading to effective tumor eradication and a successful clinical response