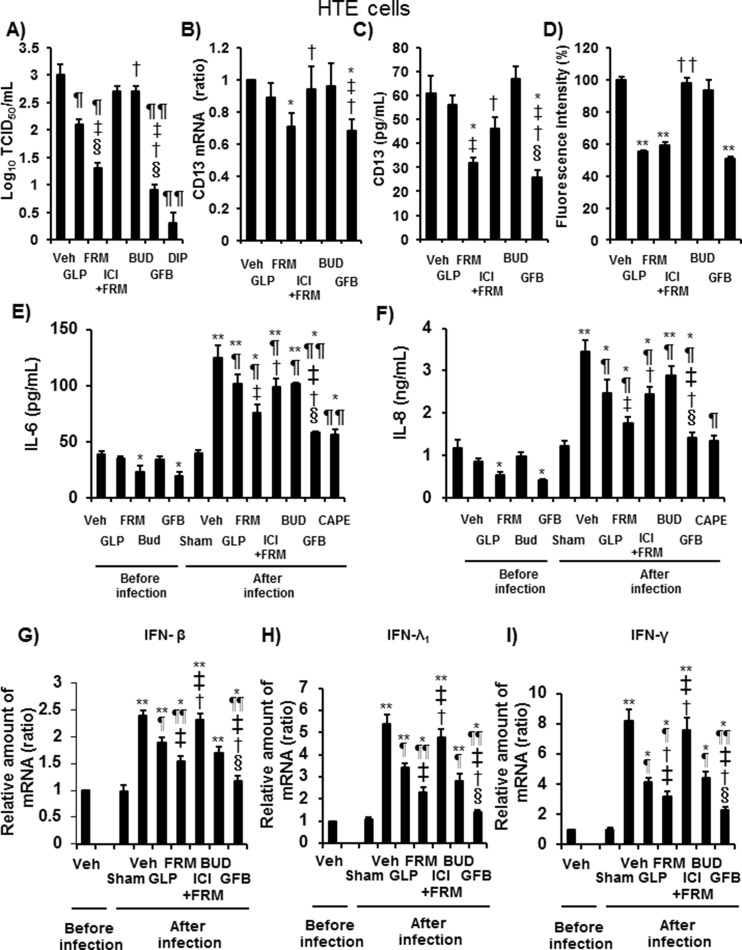
Fig. 7.
A: The viral titers in ASL collected between 24 h and 72 h after the infection of HTE cells pretreated with glycopyrronium (GLP), formoterol (FRM), the selective β2-adrenergic receptor antagonist ICI 118,551 (1 μM) plus formoterol (100 nM) (ICI + FRM), budesonide (BUD), a combination of these three drugs (GFB), the CD13 inhibitor 2′2′-dipyridyl (2.5 mM) (DIP), or vehicle (Veh). The cells were pretreated with drugs starting at 72 h before infection and lasting until the end of the experiments. The cells were pretreated with 2′2′-dipyridyl starting at 1 h before infection. B–D: CD13 mRNA (B) and protein concentrations in the ASL (C) of uninfected HTE cells and the fluorescence intensity of acidic endosomes in HTE cells (D) after pretreatment with glycopyrronium (GLP), formoterol (FRM), ICI 118,551 plus formoterol (ICI + FRM), budesonide (BUD), a combination of these three drugs (GFB), or vehicle (Veh) for 72 h. The mean value of the fluorescence intensity of the vehicle-treated cells was set to 100%. E and F: The release of IL-6 (E) and IL-8 (F) into the ASL of HTE cells pretreated with glycopyrronium (GLP), formoterol (FRM), ICI 118,551 plus formoterol (ICI + FRM), budesonide (BUD), a combination of three drugs (GFB), or vehicle (Veh) collected before and between 24 h and 72 h after infection with HCoV-229E or sham infection (Sham). G–I: The mRNA expression of IFN-β (G), IFN-λ1 (H), or IFN-γ (I) in HTE cells pretreated with glycopyrronium (GLP), formoterol (FRM), ICI plus formoterol (ICI + FRM), budesonide (BUD), a combination of the three drugs (GFB), or vehicle (Veh) extracted before and at 72 h after infection with HCoV-229E or after sham infection (Sham). A–I: The concentrations of glycopyrronium, formoterol, and budesonide were 100 nM. The results are reported as the mean ± SEM for cells of five (A-C, E-I) or seven (D) different subjects. A: Significant differences compared with the values of HTE cells after infection that were treated with vehicle, glycopyrronium, formoterol, or budesonide are indicated by ¶p < 0.05 and ¶¶p < 0.01, ‡p < 0.05, †p < 0.05, or §p < 0.01, respectively. B–D: Significant differences compared with the values of uninfected HTE cells treated with vehicle, glycopyrronium, formoterol, or budesonide are indicated by *p < 0.05 and **p < 0.05, ‡p < 0.05, †p < 0.05 and ††p < 0.01, or §p < 0.01, respectively. E–I: Significant differences compared with the values from uninfected vehicle-treated HTE cells are indicated by *p < 0.05 and **p < 0.01. Significant differences compared with the values of HTE cells after infection treated with vehicle, glycopyrronium, formoterol, or budesonide are indicated by ¶p < 0.05 and ¶¶p < 0.01, ‡p < 0.05, †p < 0.05, or §p < 0.01, respectively.