Fig. 2.
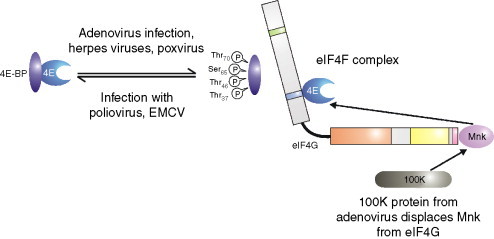
Regulation of eIF4E by 4E-BPs. The availability of eIF4E (the cap-binding protein) for eIF4F complex formation (which also contains eIF4G (the bridging protein)) and eIF4A (a deadbox helicase), is controlled by interaction with its binding partners the 4E-BPs which bind to and sequester eIF4E. The interaction of eIF4E with 4E-BP is regulated by phosphorylation and viral infection controls, either positively or negatively, the phosphorylation status of this protein. When bound to eIF4G, eIF4E can be phosphorylated by Mnk1 and it has been suggested that this may increase the affinity of eIF4E for the cap. The 100 K protein from adenovirus displaces Mnk1 from eIF4G and so prevents the phosphorylation of eIF4E.
