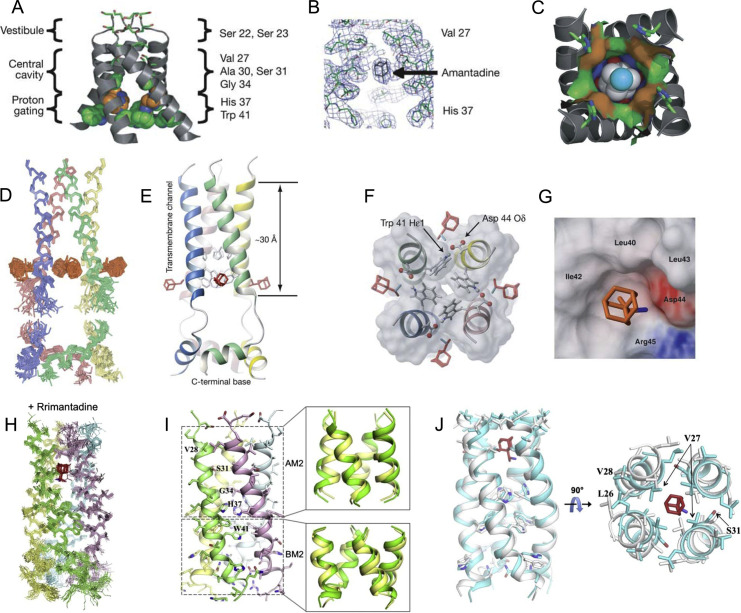
Figure 1.
P- and S-binding sites for Amt and Rim to IAV M2. (A–C) AM2 TM domain crystallized in detergent, with the most critical residues identified by site-directed mutagenesis lining the AM2 pore (A); omit map showing electron density in the Amt binding region (B); structure of amantadine (nitrogen in cyan (light gray in the print version) and carbon in white) inside the binding site showing the surface associated with residues Val-27 (red (light gray in the print version) surface), Ala-30 (green (dark gray in the print version)), Ser-31 (blue (light gray in the print version)), and Gly-34 (orange (light gray in the print version)) (C); (D and E), an ensemble of 15 low-energy structures derived from NMR restraints, with TM α-helices (residues 25–46) and amphipathic helices (residues 51–59) superimposed separately (D); ribbon representation, showing the drug Rim (colored in red (light gray in the print version)) (E); (F and G), surface representation of the Rim-binding pocket, showing the Asp-44, the indole amine of Trp-41, and Arg-45, which form the polar patch, as well as the hydrophobic wall composed of Leu-40, Ile-42, and Leu-43; (H–J) solution NMR structures of the (AM2–BM2)TM channel in the absence and presence of Rim; ensembles of 15 low-energy structures of drug-bound chimera channels. Rim is highlighted in red (light gray in the print version) (H); ribbon representation of drug-free (AM2–BM2)TM tetramer (left), and overlay of its AM2 and BM2 regions (green (dark gray in the print version)) with the corresponding regions (yellow (light gray in the print version)) of the AM2 (PDB code: 2RLF) and BM2 (PDB code: 2KIX) structures (right) (I); overlay of the drug-free (white) and the drug-bound (cyan (light gray in the print version)) chimera structures, showing substantial differences in helical packing (J).
Adapted by permission from Macmillan Publishers Ltd.: Stouffer, A. L., Acharya, R., Salom, D., Levine, A. S., Di Costanzo, L., Soto, C. S., et al. (2008). Structural basis for the function and inhibition of an influenza virus proton channel. Nature, 451(7178), 596–599. Copyright (2008); Schnell, J. R., & Chou, J. J. (2008). Structure and mechanism of the M2 proton channel of influenza A virus. Nature, 451(7178), 591–595. doi: 10.1038/nature06531. Copyright (2008). Adapted by permission from Elsevier: Pielak, R. M., Oxenoid, K., & Chou, J. J. (2011). Structural investigation of rimantadine inhibition of the AM2-BM2 chimera channel of influenza viruses. Structure, 19(11), 1655–1663. doi: 10.1016/j.str.2011.09.003. Copyright (2011).