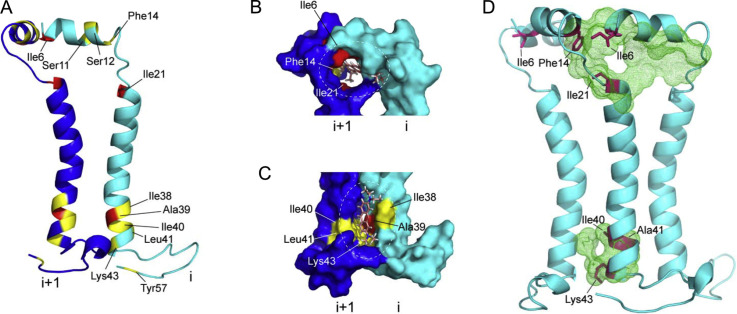
Figure 7.
Mapping of pyronin-B binding to the RSV SH protein. (A) Chemical shift perturbation (CSP) values in presence of pyronin-B, mapped onto the structure of SH protein; larger (CSP ≥ 0.07 ppm) and smaller (CSP ≥ 0.04 ppm) shifts in red (dark gray in the print version) and yellow (light gray in the print version), respectively; (B and C) two predicted pyronin-B binding sites on the SH pentamer, at the N-terminal (B) and C-terminal (C) ends of the TM domain (dotted circles). Only two monomers of SH protein pentamer (i and i + 1) are shown for simplicity; (D) druggable pockets (green (dark gray in the print version) mesh) predicted by DoGSiteScorer (Volkamer, Kuhn, Rippmann, & Rarey, 2012). The main residues that showed largest NMR chemical shift changes are shown in red (dark gray in the print version). Two monomers have been removed from the pentamer for clarity.