Fig. 1.
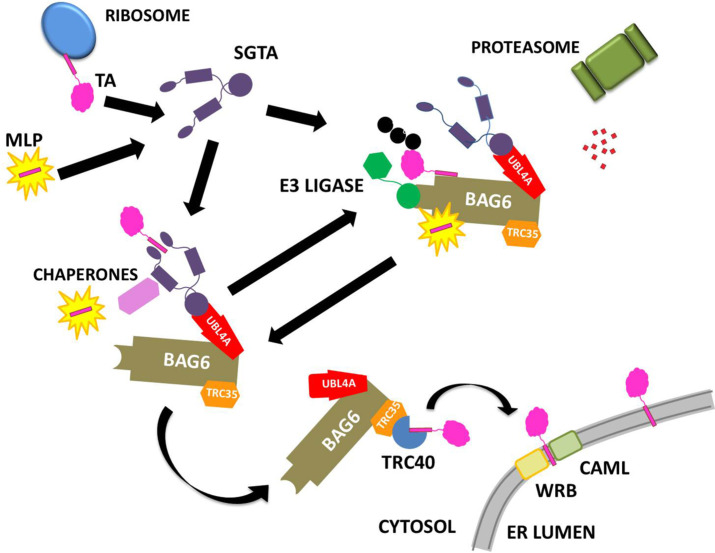
Current ideas on quality control pathways for hydrophobic proteins exposed to the aqueous cytoplasm. SGTA can catch the TMDs of newly translated TA proteins or exposed hydrophobic patches on MLPs, which bind to its C-terminal domain. In collaboration with the heterotrimeric BAG6 complex, which comprises BAG6, TRC35 and UBL4A, SGTA determines the fate of these proteins. Hydrophobic substrates bound to the BAG6 complex can be ubiquitinated by the actions of the E3 ligase RNF126 and thus targeted for proteasomal degradation. SGTA can interact with the RPN13 subunit of the 19S regulatory particle of the proteasome through its TPR domain, which has led to the proposal of an SGTA/BAG6 cycle operating at the proteasome. SGTA hands tail-anchored (TA) proteins over to TRC40 facilitating their post-translational integration into the ER by means of the transmembrane proteins WRB and CAML. Furthermore, SGTA has been implicated in hormone receptor signaling and has been associated with viral lifecycles. SGTA's interactions with Hsp70/Hsp90 chaperones via its TPR domain likely provide substrate access to additional branches of the global cellular quality control network.