Fig. 1: Structure and function of mTORC1 and mTORC2.
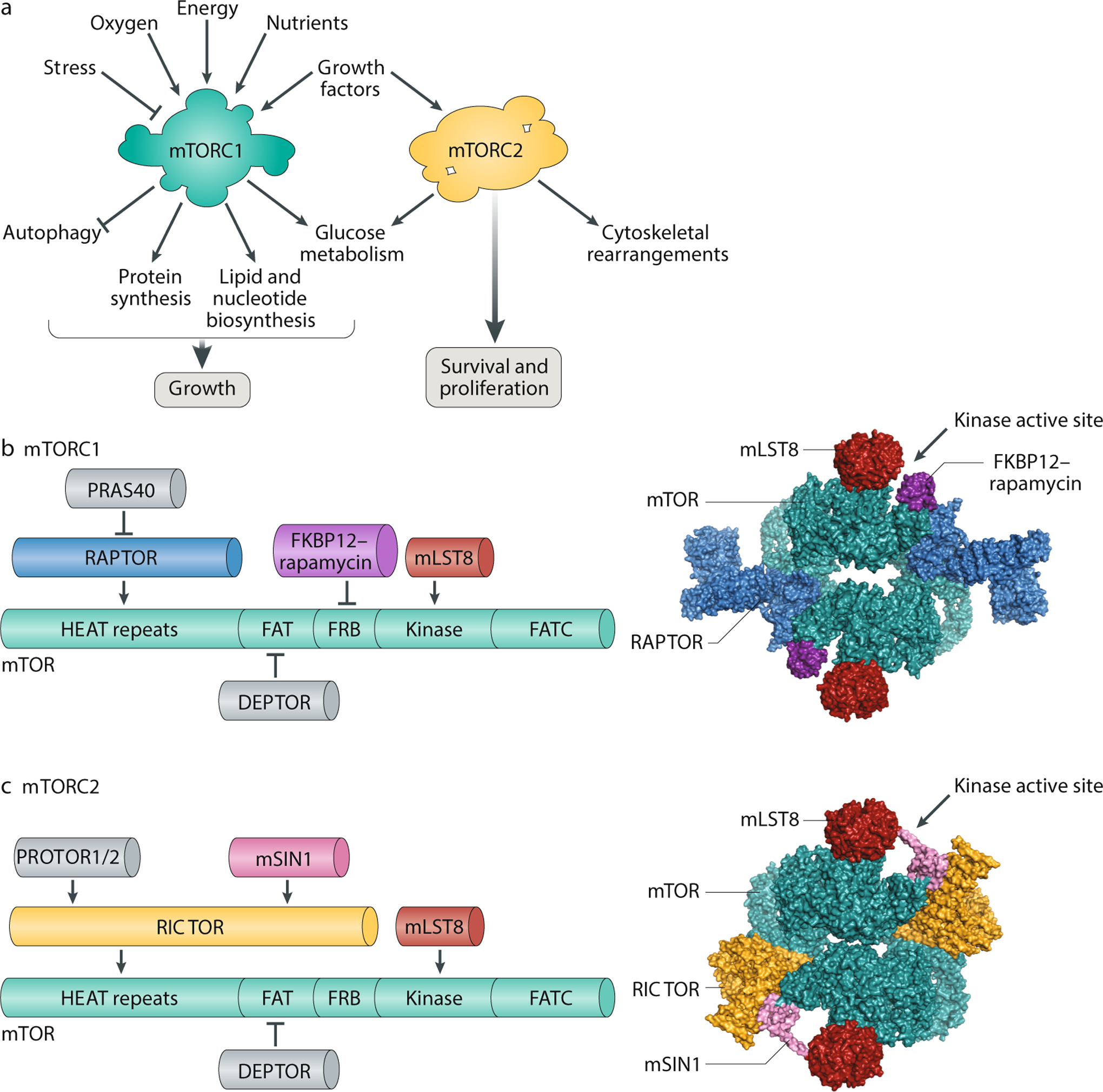
A. mTOR complex 1 (mTORC1) and mTORC2 have distinct signalling roles in the cell. mTORC1 integrates information about nutritional abundance and environmental status to tune the balance of anabolism and catabolism in the cell, while mTORC2 governs cytoskeletal behaviour and activates several pro-survival pathways. Unlike mTORC1, which is acutely inhibited by rapamycin, mTORC2 responds only to chronic rapamycin treatment. B. Components of mTORC1 (left). The domain structure of the mTOR kinase (green) is annotated with binding sites for the other mTORC1 subunits. The N-terminus of mTOR contains clusters of huntingtin, elongation factor 3, a subunit of protein phosphatase 2A and TOR1 (HEAT) repeats, followed by a FRAP, ATM and TRRAP (FAT) domain; the FKBP12–rapamycin binding (FRB) domain; the catalytic kinase domain; and the C-terminal FATC domain. mTOR binds mammalian lethal with SEC13 protein 8 (mLST8), a core component of the complex, and DEP-domain-containing mTOR-interacting protein (DEPTOR), an endogenous inhibitor of mTORC1 activity. Regulatory-associated protein of mTOR (Raptor), the defining subunit of mTORC1, binds mTOR with its own HEAT repeats and is required for lysosomal localization of the complex. Raptor also recruits proline-rich AKT substrate 40 kDa (PRAS40), an insulin-regulated inhibitor of mTORC1 activity. A 5.9-Å reconstruction of mTORC1 (without PRAS40 and DEPTOR) complexed with FKBP12–rapamycin is shown as a surface representation (Protein Database (PDB) ID: 5FLC) (right). C. Components of mTORC2 (left). The mTOR kinase (green) is annotated with the binding sites for the other constituent subunits of mTORC2. These subunits include mLST8, DEPTOR and RICTOR, the defining component of mTORC2. As a scaffolding protein, RICTOR recruits protein associated with rictor 1 or 2 (PROTOR1/2) to the complex, along with MAPK-interacting protein (mSIN1), which contains a pleckstrin homology domain. A 4.9-Å reconstruction of mTORC2 (without DEPTOR and PROTOR) is shown as a surface representation (PDB: 5ZCS) (right).
