Fig. 3: Upstream regulators of the mTOR signalling pathway.
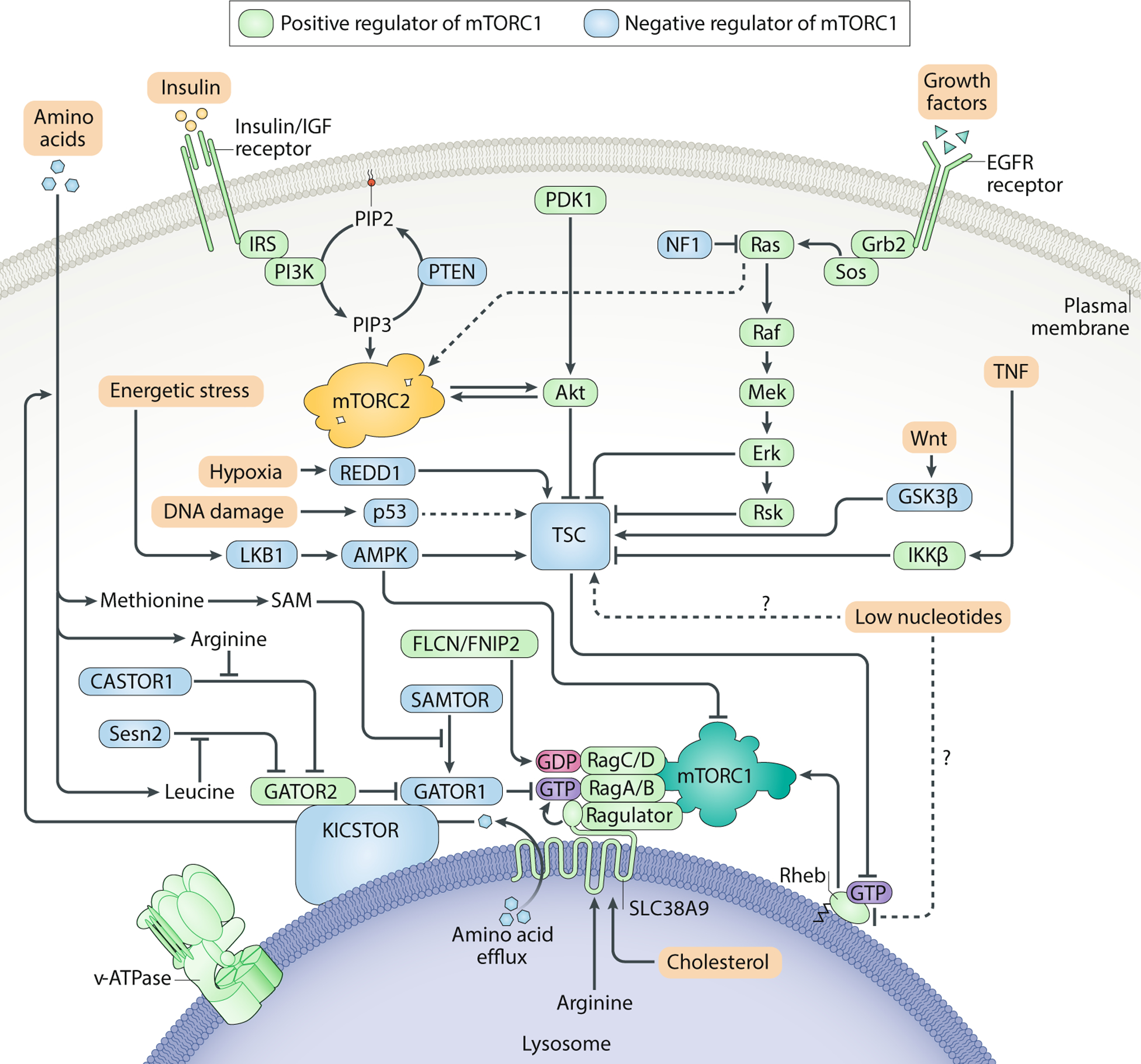
mTOR complex 1 (mTORC1) and mTORC2 integrate upstream environmental information to gate their own activation. Because mTORC1 controls cellular entry into an anabolic state that requires copious amounts of energy and macromolecules, activation of the complex should only occur when amino acids, insulin/growth factors, ATP and oxygen are all readily available. To ensure that all of these requirements are satisfied, mTORC1 must translocate to the lysosome by anchoring onto the Rag GTPases, which are only competent to recruit mTORC1 in the presence of amino acids. Once localized to the lysosomal surface, mTORC1 can be then be activated by the small GTPase Rheb in its GTP-bound state. Importantly, GTP loading of Rheb is promoted by growth factors and opposed by energetic stress or hypoxia. All of these inputs converge on tuberous sclerosis complex (TSC), which acts as a GAP for Rheb. mTORC2 is thought to be primarily regulated by growth factors. Although it is not clear where mTORC2 activation occurs, the pleckstrin homology domain on MAPK-interacting protein 1 (mSIN1) may recruit mTORC2 to the plasma membrane. Positive regulators of the mTORC1 pathway are shown in green, while negative regulators of mTORC1 are shown in blue. AMPK, AMP-activated protein kinase; CASTOR, cellular arginine sensor for mTORC1; EGFR, epidermal growth factor receptor; FLCN, folliculin; GATOR, GAP activity towards the Rags; Grb2, growth factor receptor-bound protein 2; GSK3, glycogen synthase kinase 3; IGF, insulin-like growth factor; IKKβ, inhibitor of nuclear factor κB kinase β; IRS, insulin receptor substrate; LKB1, liver kinase B1; Mek, MAPK/ERK kinase; NF1, neurofibromatosis type 1; PIP2, phosphatidylinositol (4,5)-bisphosphate; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; PTEN, phosphatase and tensin homologue; RSK, p90 ribosomal S6 kinase; SAM, S-adenosylmethionine; SAMTOR, S-adenosylmethionine sensor; Sos, son of sevenless; TNF, tumour necrosis factor.
