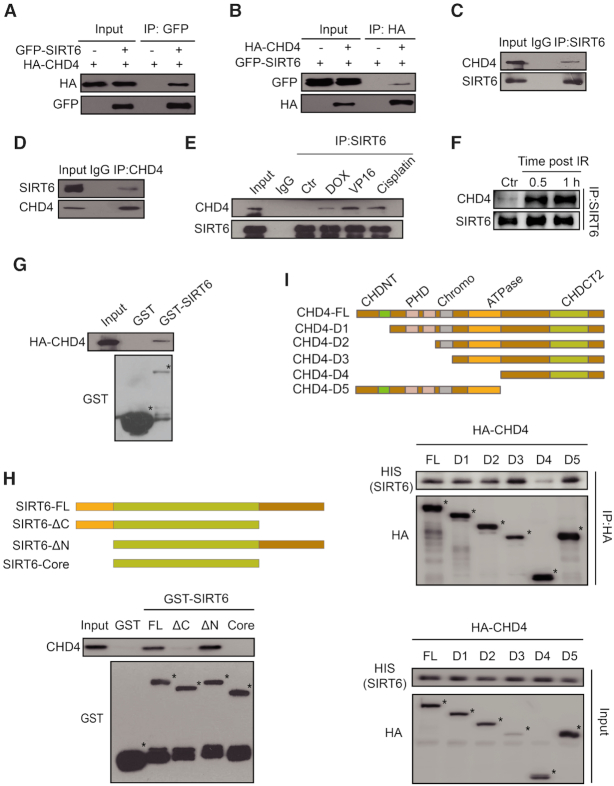
Figure 1.
The interaction between SIRT6 and CHD4 markedly increases in response to DNA damage. (A) Whole-cell lysates of HEK293T cells transfected with HA-CHD4 with or without GFP-SIRT6 transfection were precipitated with an anti-GFP antibody and analyzed by western blotting, as indicated. (B) Whole-cell lysates of HEK293T cells transfected with GFP-SIRT6 with or without HA-CHD4 transfection were precipitated with an anti-HA antibody and analyzed by western blotting, as indicated. (C andD) Nuclear proteins from HCT116 cells were extracted and immunoprecipitated using an anti-SIRT6 (C) or an anti-CHD4 (D) antibody. Rabbit IgG was used as a negative control. Western blotting was performed with the indicated antibodies. (E) HCT116 cells were treated with 1 μM doxorubicin (DOX) /40 μM etoposide (VP16)/10 μM cisplatin for 1 h and cell extracts were then precipitated with an anti-SIRT6 antibody before western blotting with the indicated antibodies. (F) HCT116 cells were exposed to 10 Gy IR and released for 0.5 or 1 h. The cell extracts were then precipitated with an anti-SIRT6 antibody and western blotting was performed with the indicated antibodies. (G) GST-SIRT6 proteins were purified and incubated with HCT116 whole-cell lysates that expressed HA-CHD4, and analyzed by western blotting. (H) GST-fusion proteins of full length (FL) SIRT6 and SIRT6 fragments were purified and incubated with HCT116 whole cell lysates, and analyzed by western blotting. (I) HA-fragments of CHD4 were infected into HCT116 cells. The cell extracts were incubated with HIS-SIRT6 proteins and then analyzed by western blotting.