Figure 4.
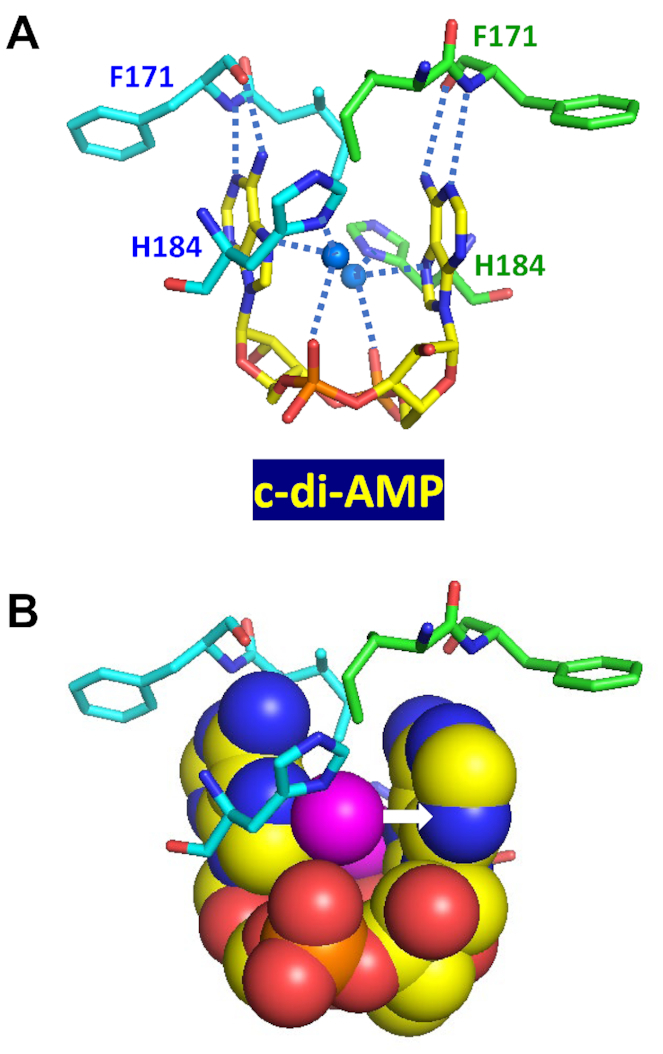
Binding of c-di-AMP to CpaA. Complex of U-shaped c-di-AMP with two RCK_C domains of S. aureus K+/H+ antiporter CpaA (PDB: 5F29) (99) shows each water molecule (solid blue circles in (A) and magenta spheres in (B)) coordinated by the N7 atom and phosphate oxygen atom of c-di-AMP, as well as the N atom from the side chain of His184. The lone pair electrons of oxygen atom form a water-mediated lone pair–adenine interaction (indicated by a white arrow in B). The N1 and N6 atoms of c-di-AMP adenines bind the backbone nitrogen and carbonyl oxygen atoms of Phe171, respectively, of both RCK_C domains.
