Fig. 7.
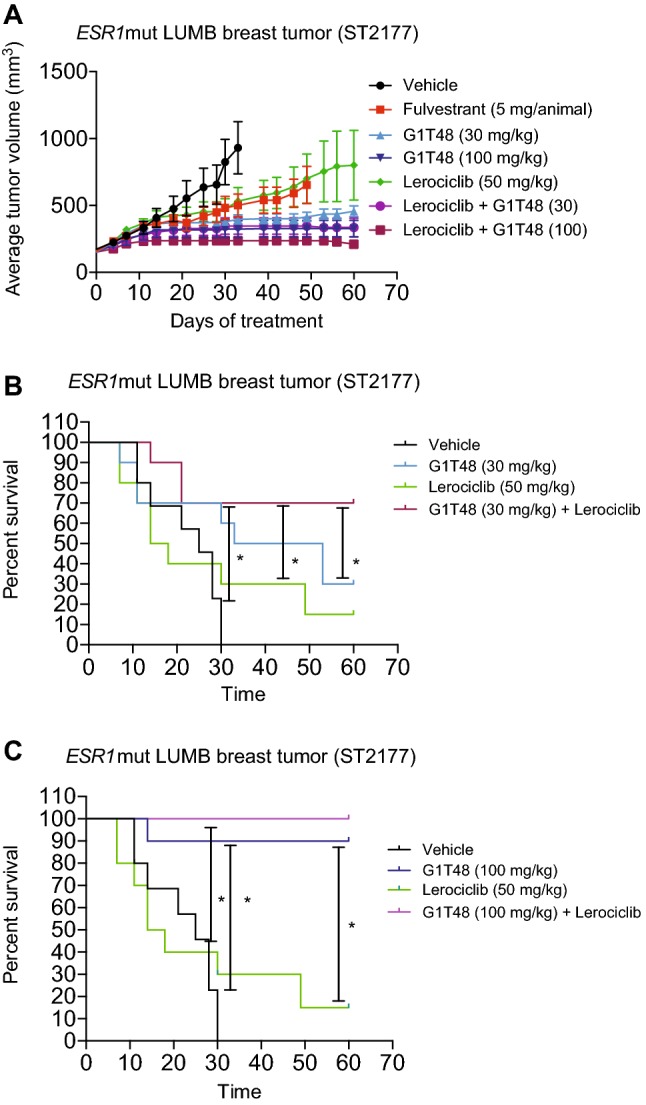
Evaluation of the combined efficacy of lerociclib and G1T48 in a Patient-Derived Xenograft Model harboring the ER-Y537S Mutation. a Female nu/nu mice were engrafted with a START Patient-Derived Xenograft Model (START-PDX) model, designated ST2177, harboring an ER-Y537S mutation. Mice were randomized to vehicle, fulvestrant (5 mg/animal), G1T48 (30 or 100 mg/kg), lerociclib (50 mg/kg), or the combination of G1T48 and lerociclib and treated for 60 days. b, c Kaplan–Meier analysis is presented as time for tumors to reach endpoint (2.5 times original tumor volume). *Kaplan–Meier analysis followed by a Mantel–Cox test for significance demonstrated significantly greater tumor growth delay for these comparisons using an adjusted Bonferroni threshold of p < 0.012. Error bars represent SEM
