Abstract
5-Enolpyruvylshikimate 3-phosphate synthase (EPSPS) is the primary target for the broad-spectrum herbicide, glyphosate. Improvement of EPSPS gene for high level of glyphosate tolerance is important to generate glyphosate-tolerant crops. In this study, we report the isolation and characterization of EPSPS genes of glyphosate-tolerant Pseudomonas nitroreducens strains FY43 and FY47. Both P. nitroreducens strains FY43 and FY47, which showed glyphosate tolerance up to 8.768% (518.4 mM, 32 × higher than field application), were isolated from soil samples collected from oil palm plantation with a long history of glyphosate application. The glyphosate tolerance property of EPSPS genes of strains FY43 and FY47 was functionally characterized by expressing the genes in Escherichia coli strain BL21(DE3). Error-prone PCR was performed to mutagenize native EPSPS gene of strains FY43 and FY47. Ten mutagenized EPSPS with amino acid changes (R21C, N265S, A329T, P71L, T258A, L184F, G292C, G292S, L35F and A242V) were generated through error-prone PCR. Both native and mutated EPSPS genes of strains FY43 and FY47 were introduced into Escherichia coli strain BL21(DE3) and transformants were selected on basal salt medium supplemented with 8.768% (518.4 mM) glyphosate. Mutants with mutations (R21C, N265S, A329T, P71L, T258A, L35F, A242V, L184F and G292C) showed sensitivity to 8.768% glyphosate, whereas glyphosate tolerance for mutant with G292S mutation was not affected by the mutation.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02176-7) contains supplementary material, which is available to authorized users.
Keywords: Pseudomonas nitroreducens, Glyphosate, MLSA, Anib, GGDC
Introduction
5-Enolpyruvylshikimate 3-phosphate synthase (EPSPS; 3-phosphoshikimate 1-carboxyvinyl-transferase) is an essential enzyme in the shikimate pathway, which is responsible for the production of aromatic amino acids (tryptophan, phenylalanine, tyrosine) and secondary metabolites. EPSPS converts phosphoenolpyruvate (PEP) and shikimate-3-phosphate (S3P) to 5-enolpyruvylshikimate-3-phosphate (EPSP). Glyphosate (N-[phosphonomethyl]-glycine), a broad-spectrum herbicide, inhibits EPSPS leading to plant death (Bartel 1997). Glyphosate has been widely used for weed management in agricultural fields (Gonzini et al. 1999). Two classes of EPSPS (Class I and Classs II) have been identified in plants and bacteria. Both classes of EPSPS shared less than 50% amino acid sequence similarity (Zhou et al. 2006). EPSPS, derived mainly from many plants and Escherichia coli, were classified as Class I EPSPS (glyphosate-sensitive), whereas glyphosate-tolerant EPSPS which is found in various bacteria including Agrobacterium sp. strain CP4, Pseudomonas sp. strain PG2982, Staphylococcus aureus and S. pneumoniae were termed Class II EPSPS (Funke et al. 2007).
Several bacterial strains were tested for glyphosate tolerance studies and some were found as glyphosate-tolerant bacteria in previous studies (Manogaran et al. 2017). Burkholderia gladioli and Pseudomonas oryzihabitans were isolated from soybean plants cultivated in soil and subjected to glyphosate sensibility evaluation. Burkholderia gladioli were able to grow in medium containing 20 mM glyphosate. whereas the growth of Pseudomonas oryzihabitans was fully inhibited in 20 mM glyphosate (Kuklinsky-Sobral et al. 2005). Burkholderia vietnamiensis and Burkholderia sp. AQ5–12 were isolated from water and soil specimen in Malaysia, and they can tolerate up to 12 mL/L of glyphosate (Manogaran et al. 2017). On the other hand, Acetobacter sp. was found from rice field in Nigeria and no growth inhibition was observed at 100–250 g/L of glyphosate, indicating Acetobacter sp. Was able to tolerate up to 250 g/L of glyphosate (Moneke et al. 2010). Salmonella Typhimurium, Salmonella Enteriditis and Salmonella Gallinarum were found to have glyphosate resistance up to 5 g/L (Shehata et al. 2013). Besides, Kryuchkova et al (2013) mentioned that Comamonas sp. K4, Azomonas sp. K5, Enterobacter cloacae K7 and Alcaligenes sp. K1 were resistant up to 10 mM of glyphosate. Pseudomonas sp. with different levels of glyphosate tolerance have been identified (Jacob et al. 1988; Liang et al. 2008; Moneke et al. 2010; Pollegioni et al. 2011; Benslama and Boulahrouf 2013; Kryuchkova et al. 2013; Aristilde et al. 2017).
The identification of novel EPSPS gene from different source or improvement of existing EPSPS gene is important for developing glyphosate-resistant crops that confer better resistance toward glyphosate. Fitzgibbon and Braymer (1990) found that recombinant E. coli harboring glyphosate-resistant gene of Pseudomonas sp. strain PG2982 showed the ability to grow in the media containing 100 mM glyphosate. Besides, several glyphosate-tolerant EPSPS mutants [G96A from E.coli (Eschenburg et al. 2002); P101S from Salmonella typhimurium (Stalker et al. 1985); P106S from goosegrass, Eleusine indica (Baerson et al. 2002)] were reported previously and all displayed improvement in growth with the addition of glyphosate. In vitro directed evolution through error-prone PCR has been widely used for screening of novel functional region within a sequence (Ling et al. 1991). The EPSPS gene mutant (P106L) from Oryza sativa was created by error-prone PCR and showed threefold increase in glyphosate resistance as compared to wild-type EPSPS (Zhou et al. 2006). He et al. (2003) found the T42M mutant from EPSPS gene of E. coli and Salmonella typhimurium based on directed evolution strategy; an increase in glyphosate resistance and enzyme activity were observed. The EPSPS gene from Pseudomonas stutzeri A1501, mutagenized through error-prone PCR and single amino acid substitution (A130S), was obtained and resulted in increased resistance to 200 mM glyphosate (Liang et al. 2008).
In our previous study, Pseudomonas nitroreducens strains FY43 and FY47 were isolated from an oil palm plantation at Sungai Siput, Perak, Malaysia, where the field has long-term glyphosate application history (Looi 2016). However, no detailed study was conducted on the EPSPS genes of these strains. Thus, we aim to functionally characterize the EPSPS genes of P. nitroreducens strain FY43 and FY47 through error-prone PCR approach.
Materials and methods
Bacterial strains and cultivation
Pseudomonas nitroreducens strain FY43 and FY47 were isolated from an oil palm plantation at Sungai Siput, Perak, Malaysia (Looi 2016), where the field has a history of long-term glyphosate usage for weed control. The bacterial strains were cultured in Lysogeny broth (LB broth) (BD Difco™, USA) and maintained in 20% glycerol in − 80 °C freezer (Thermo Fisher Scientific, USA) located at High Impact Research building, University of Malaya.
16S rRNA sequencing
16S rRNA gene sequence was amplified from bacterial culture of Pseudomonas nitroreducens strain FY43 and FY47 using universal 16S rRNA primers, 24F (5′-AGA GTT TGA TCC TGG CTC AG-3′) and 1492R (5′-TAC GGY TAC CTT GTT ACG ACT T-3′). PCR program consists of an initial denaturation of 95 °C for 3 min, followed by 30 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, extension at 72 °C for 2 min, and finally an extension of 72 °C for 5 min. The PCR product was gel purified using the NucleoSpin® Gel and PCR Clean-up kit (MACHEREY–NAGEL GmbH, Germany) to remove unspecific products. Then the purified PCR product was sequenced by Apical Scientific Sdn. Bhd. Sequencing results were searched against 16S rRNA sequences deposited in the National Center for Biotechnology Information (NCBI) database using nucleotide BLAST (BLASTN) algorithm.
Phylogenetic analysis of Pseudomonas nitroreducens strain FY43 and FY47 based on 16S rRNA gene
The 16S rRNA gene sequence of Pseudomonas nitroreducens strain FY43 and FY47 were aligned together with eight P. nitroreducens, three P. aeruginosa, three P. syringae, three P. pavonaceae, three P. viridiflava, three P. fuscovaginae and three P. avellanae using ClustalW embedded in MEGA program version 7.0 (Kumar et al. 2016). The phylogenetic analysis was performed using neighbor-joining (NJ) algorithm with 1000 bootstrap replicates. Evolutionary distances were computed using the Jukes–Cantor method.
Glyphosate resistance assay
Both Pseudomonas nitroreducens strains FY43 and FY47 were sub-cultured in fresh LB broth (BD Difco™, USA) at 37 °C, 200 rpm for overnight. The overnight-grown cultures were centrifuged at 5000 rpm for 1 min to pellet down the bacteria cells. The supernatant was discarded using micropipette and the pellets were resuspended in either fresh LB broth or 1× basal salt media consisting 5.8 g/L K2HPO4, 4.5 g/L KH2PO4, 2 g/L (NH4)2SO4, 1 mg/L FeSO4·7H2O, 0.16 g/L MgCl2·2H2O, 20 mg/L CaCl2, 2 mg/L Na2MoO4 and 1 mg/L MnCl2.
The bacterial cells were transferred to LB broth and basal salt media supplemented with 0.137%, 0.274%, 0.548%, 1.096%, 2.192%, 4.384%, 8.768% and 17.536% of glyphosate (Farmcochem Sdn. Bhd., Malaysia). Various percentages of glyphosate were used to determine the maximum glyphosate tolerance level of the bacteria. The initial OD600 of the bacteria strains was maintained at 0.1. LB broth, and basal salt media without glyphosate were used as the controls. The cultures were incubated at 37 °C with agitation at 200 rpm. The OD600 reading of the culture was recorded at three time-points: 0, 48 and 120 h. All the samples and measurements were conducted in triplicate. The growth rate was calculated according to the equation suggested by Widdel (2010).
Error-prone PCR amplification and Gateway® cloning of EPSPS gene
Five rounds of error-prone PCR were conducted to introduce point mutations to the native EPSPS gene. PCR was performed using 2 µL of 10 × iTaq buffer (Bio-Rad, CA), 2 µL of PCR Nucleotide Mix [10 mM each] (Bio-Rad, CA), 1.0 µL of each primers attB1-EPSPS (5′-AAA AAG CAG GCT CCA TGC ACA ACA AYG ACC TGA T-3′) and attB2-EPSPS (5′-AGA AAG CTG GGT GTC AGT TYT TCT CCT CRG CGA C-3′) [10 µM each], 0.5 µL of 5 U/µL iTaq DNA polymerase (Bio-Rad, CA), and 4 µL of purified PCR product of native EPSPS gene in a total reaction volume of 20 µL. The PCR program consists of an initial denaturation of 95 °C for 2 min, followed by 30 cycles of denaturation at 94 °C for 20 s, annealing at 60 °C for 30 s, and extension at 72 °C for 1.5 min. Rounds 2–5 of Step-1 PCR were performed using 10 µL of the previous round of the PCR product as the template.
Native and mutated EPSPS genes were cloned into Gateway™ pDEST14™ (Thermo Fisher Scientific, USA) vector using Gateway system (Thermo Fisher Scientific, USA) and introduced into the Top10 chemically competent E. coli cells (Thermo Fisher Scientific, USA) using the heat-shock method (Froger and Hall 2007). Plasmids were isolated from positive transformants using FavorPrep Plasmid Extraction Mini Kit (Favorgen Biotech Corp, USA) according to manufacturer’s instructions. Purified plasmids were sequenced by Apical Scientific Sdn. Bhd. EPSPS sequence integrity was checked by aligning the mutated genes to the native EPSPS genes of strains FY43 and FY47 using ClustalW Multiple alignment software embedded in BioEdit version 7.2.5 (Tom Hall Ibis Biosciences 1997).
Transformation of mutated EPSPS genes and glyphosate assay of recombinant E.coli BL21 (DE3)
Purified plasmids harboring native or mutated EPSPS genes were introduced into E. coli BL21 (DE3) competent cells using the heat shock method (Froger and Hall 2007). The control plasmid was prepared by transforming the empty vector to BL21 (DE3) chemically competent cell (TransGen Biotech, China). All the transformants were plated on antibiotic (50 mg/L Ampicillin) selection plates and incubated at 37 °C for overnight. A single colony was resuspended in 5 mL of LB broth with ampicillin, incubated at 37 °C until OD600 reading reached 0.4–0.8. The bacterial culture was then induced with 0.5 mM IPTG at 37 °C for 3 h. The transformants were then subjected to glyphosate assay to compare the growth rate of native and mutated EPSPS genes in E. coli BL21 (DE3) under the exposure of glyphosate. The transformants were transferred to LB broth and basal salt media supplemented with 8.768% glyphosate (Farmcochem Sdn. Bhd., Malaysia). The initial OD600 reading of the bacteria strains was maintained at 0.1. The cultures were incubated at 37 °C with agitation at 200 rpm. The OD600 reading of the culture was recorded at two time-points: 0 and 48 h. All the samples and measurements were conducted in triplicate. The growth rate was calculated according to the equation suggested by Widdel (2010). The one-way analysis of variance (ANOVA) was used by using SPSS software version 25 to determine any statistically significant differences between the means of growth rates for all transformants under exposure to glyphosate.
Results
Molecular identification of P. nitroreducens strain FY43 and FY47
The 16S rRNA gene sequences of Pseudomonas sp. FY43 and Pseudomonas sp. FY47 were amplified and compared to 16S rRNA sequences deposited in the National Center for Biotechnology Information (NCBI) database. Phylogenetic analysis showed that both Pseudomonas sp. FY43 and FY47 clustered together with other P. nitroreducens (Fig. 1). This indicates that both bacteria belong to P. nitroreducens species. They are designated as Pseudomonas nitroreducens strain FY43 and Pseudomonas nitroreducens strain FY47.
Fig. 1.
The phylogenetic tree was constructed from the 16S rRNA gene sequences using the Jukes–Cantor and neighbor-joining method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The scale bar represents five nucleotides exchange per 1000 nucleotides
Growth reaction of P. nitroreducens strain FY43 and FY47 to glyphosate
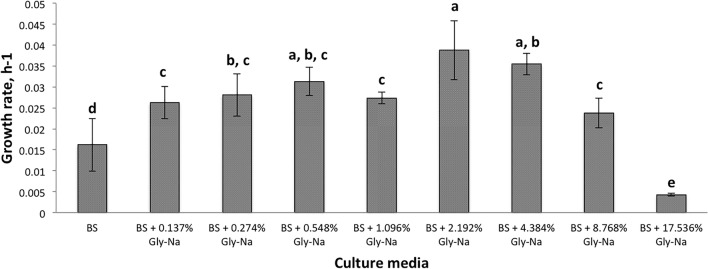
The glyphosate tolerance level of Pseudomonas nitroreducens strains FY43 and FY47 were evaluated using basal salt (BS) media supplemented with various concentrations of glyphosate. The LB medium was used as the control in this study, where the aromatic amino acids needed for the growth of both strains were supplied by the LB media (Supplementary Fig. 1 and 2). All the growth rates of P. nitroreducens strain FY43 in the BS media supplemented with different concentrations of glyphosate-sodium were significantly different compared to BS alone after 48 h of incubation (p = 0.00; Fig. 2). A similar observation was observed after 120 h of incubation in the BS media supplemented with different concentrations of glyphosate-sodium (Supplementary Fig. 3). Pseudomonas nitroreducens strain FY47 incubated in BS media with 0.137%, 8.768% and 17.536% glyphosate-sodium showed significant difference in the growth rate compared to BS alone after 48 h of incubation (p = 0.00; Fig. 3). All the growth rates of P. nitroreducens strain FY47 showed significant difference in the BS media supplemented with different glyphosate-sodium concentrations compared to BS media alone, except media with 0.274% glyphosate (Supplementary Fig. 4). The growth rate of both strains was reduced significantly when incubated in BS supplemented with 17.536% glyphosate-sodium (Figs. 2, 3; Supplementary Fig. 3 and 4). This indicates that both P. nitroreducens strain FY43 and FY47 can tolerate up to 8.768% glyphosate.
Fig. 2.
The growth rate of Pseudomonas nitroreducens strain FY43 in BS media alone and BS media with different concentrations of glyphosate. The growth rate was measured at 48 HPI. The vertical bars represent the standard deviation of the mean (n = 3). Different letter indicates significant differences at 95% by Duncan’s comparison test. All the growth rates of P. nitroreducens strain FY43 in the BS media supplemented with different concentrations of glyphosate-sodium were significantly different compared to BS alone
Fig. 3.
Growth rate of Pseudomonas nitroreducens strain FY47 in BS media and BS media with different concentrations of glyphosate. The growth rate was measured at 48 HPI. The vertical bars represent the standard deviation of the mean (n = 3). Different letter indicates significant differences at 95% by Duncan’s comparison test. The growth rates of Pseudomonas nitroreducens strain FY47 in BS media with 0.137%, 8.768% and 17.536% glyphosate-sodium were significantly different compared to BS alone
Sequence analysis of native EPSPS genes of Pseudomonas nitroreducens strain FY43 and FY47
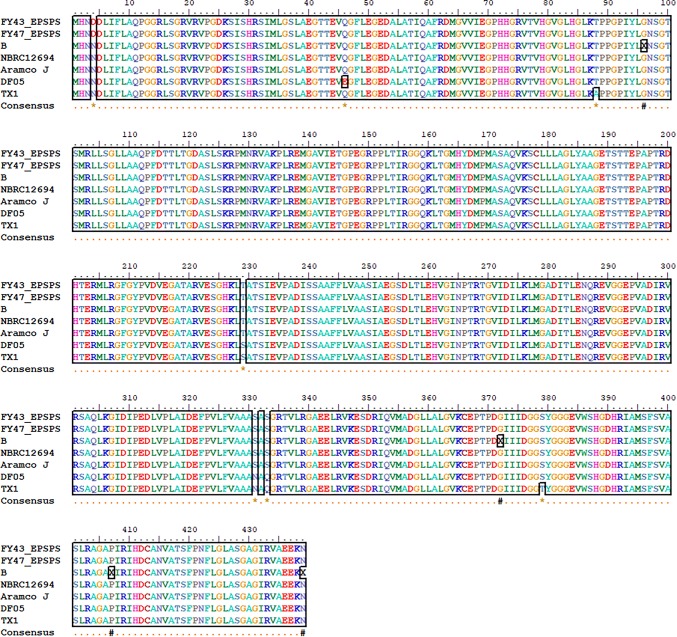
Native EPSPS genes of P. nitroreducens strains FY43 and FY47 were amplified and sequenced. Multiple sequence alignment analysis showed that EPSPS genes extracted from Pseudomonas nitroreducens strain FY43 and FY47 assembled genomes shared high homology to Pseudomonas nitroreducens strain NBRC12694, Aramco J, B, TX01 and DF05 with few amino acid changes (Fig. 4). EPSPS of P. nitroreducens strains NBRC12694 and Aramco J shared the highest amino acid sequence homology to EPSPS of P. nitroreducens strain FY43 and FY47 with only one amino acid change at position 4 (arrowhead; Fig. 4). Four deletions were detected in the ESPSP gene of P. nitroreducens strain B (star, Fig. 4) and a gap was introduced to each deleted position to reconstitute the gene. Seven amino acid changes were detected when comparing EPSPS of strain FY43 and FY47 to P. nitroreducens strain DF05 and TX1 (arrowhead and arrows; Fig. 4).
Fig. 4.
Multiple sequence alignment of the EPSPS gene. The stars represent the position of deletion detected in strain B. A gap was then introduced in each position to reconstitute the amino acid sequence of strain B EPSPS gene
Generation of EPSPS gene mutants
Sequence analysis of both native and mutated EPSPS genes using ClustalW multiple alignment program (BioEdit version 7.2.5; Tom Hall Ibis Biosciences 1997) detected single nucleotide substitutions at various positions of the genes (Table 1). In total, 16 single nucleotide substitutions were identified after two rounds of error-prone PCR and screening. Among all, only ten amino acid substitutions were observed, and the remaining six nucleotide changes showed no amino acid substitutions as compared to the native EPSPS gene. More than one mutation site was observed in some of the mutated EPSPS gene, FY43M-4, FY43M-8, FY47M-6 and FY47M-9 (Table 1).
Table 1.
Nucleotide changes of mutated EPSPS gene sequence obtained from error-prone PCR as compared to non-mutated EPSPS gene sequence
| Sample | Nucleotide substitution | Position in nucleotide sequence | Amino acid substitution |
|---|---|---|---|
| FY43M-4 |
C → T C → T A → G G → A |
61 273 794 985 |
R21C _ N265S A329T |
| FY43M-8 |
C → T C → T |
15 212 |
_ P71L |
|
FY47M-1 FY47M-2 FY47M-3 |
A → G A → G A → G |
772 772 772 |
T258A T258A T258A |
| FY47M-6 |
T → C C → T G → T |
12 550 874 |
_ L184F G292C |
| FY47M-7 | G → A | 874 | G292S |
| FY47M-8 | T → C | 12 | _ |
| FY47M-9 |
T → C C → T C → T C → T |
12 103 725 843 |
_ L35F A242V _ |
To investigate which amino acid plays an important role in the glyphosate tolerance property of EPSPS gene, mutants were synthesized using the error-prone PCR method. Native and mutant EPSPS genes were then transformed into E. coli BL21(DE3) and incubated in BS media supplemented with 8.768% of glyphosate, respectively. Recombinant E. coli BL21(DE3) with only empty vector was included as control in the glyphosate assay.
When incubated in BS media, the growth rates of FY43M-4 (R21C, N265S, and A329T; Table 1) and FY47M-9 (L35F and A242V; Table 1) mutants that harbored three and two amino acid substitutions were reduced by 0.008342472 ± 0.004289985 h−1 (p = 0.000) and 0.01259636 ± 0.007432156 h−1 (p = 0.005), respectively, as compared to control, growth rate at 0.0161 h−1 (Supplementary Fig. 5). On the other hand, the growth rate of FY43M-8 (P71L; Table 1), FY47M-6 (L184F, G292C; Table 1) and FY47M-7 (G292S; Table 1) mutants showed 0.01002545 ± 0.004349684 h−1 (p = 0.038), 0.015045544 ± 0.003225448 h−1 (p = 0.001) and 0.009195559 ± 0.005644615 h−1 (p = 0.000) improvement compared to the control after 48 h of incubation in BS media (Supplementary Fig. 5). No significant difference was observed for native EPSPS FY43 and FY47M-2 (T258A; Table 1) mutants (Supplementary Fig. 5).
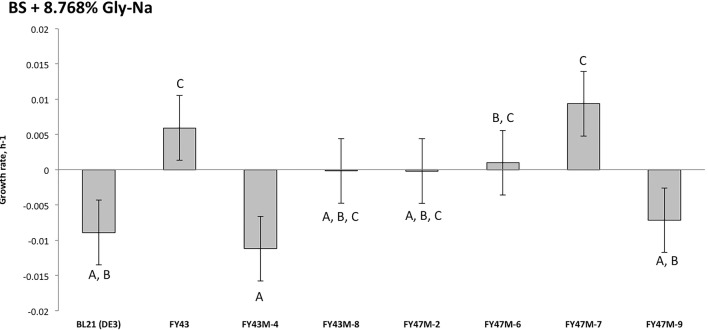
The control BL21(DE3) showed sensitivity to 8.768% glyphosate (Fig. 5). Interestingly, EPSPS mutants [FY43M-4 (R21C, N265S, and A329T; Table 1), FY43M-8 (P71L; Table 1), FY47M-2 (T258A; Table 1), FY47M-6 (L184F, G292C; Table 1), FY47M-9 (L35F and A242V; Table 1)] also showed sensitivity to the dosage of glyphosate used where their growths were fully compromised (Fig. 5). Glyphosate tolerance of recombinant E.coli BL21(DE3) with native FY43 EPSPS gene and FY47M-7 (G292S; Table 1) mutant were maintained where their growth rates were improved by 0.014848798 ± 0.003247253 h−1 (p = 0.033), and 0.018269967 ± 0.012137225 h−1 (p = 0.028), respectively, as compared to the control (Fig. 5). This indicates that all the amino acid substitutions found in this study except G292S affected the tolerance of FY43 EPSPS to glyphosate.
Fig. 5.
The growth rate of the control plasmid [E.coli BL21(DE3)], the host (Pseudomonas nitroreducens strains FY43 and FY47), recombinant E.coli BL21(DE3) with native and mutant EPSPS gene in basal salt (BS) media supplemented with 8.768% of glyphosate-sodium herbicide after 48 h of incubation. The vertical bars represent the standard deviation of the mean (n = 3). Different letters indicate significant differences at 95% by Duncan’s comparison test. All EPSPS mutants showed sensitivity to 8.768% glyphosate except FY47M-6 and MY47M-7. Wild-type EPSPS (FY43) showed tolerance to 8.768% glyphosate
Discussion
Two soil bacteria were isolated from oil palm plantation that has long-term glyphosate application history. Both strains were identified through 16S rRNA sequencing and classified as Pseudomonas nitroreducens species, namely Pseudomonas nitroreducens strains FY43 and FY47. Glyphosate assay showed that the growth rates of both Pseudomonas nitroreducens strains FY43 and FY47 were generally improved in BS media supplemented with different concentrations of glyphosate (except for media with 17.536% glyphosate) as compared to those incubated in BS media alone. This suggests that both Pseudomonas nitroreducens strain FY43 and FY47 are glyphosate-tolerant bacteria. Pseudomonas nitroreducens strains FY43 and FY47 were able to survive in the media supplemented with up to 8.768% (equivalent to 518.4 mM) glyphosate, whereas in other studies Pseudomonas stutzeri strain A1501 (Liang et al. 2008), Pseudomonas sp. strain LBr (Jacob et al. 1988), Pseudomonas sp. strain K3 (Kryuchkova et al. 2013), Pseudomonas putida (Benslama and Boulahrouf 2013) and Pseudomonas fluorescens (Moneke et al. 2010) were only tolerant up to 200 mM, 20 mM, 10 mM, 9 g/L and 250 g/L of glyphosate, respectively.
Error-prone PCR approach has been applied to functionally characterize and improve the glyphosate tolerance property of EPSPS gene in various organisms (Zhou et al. 2006). In this study, we conducted error-prone PCR on EPSPS genes from Pseudomonas nitroreducens strains FY43 and FY47 to functionally characterize the EPSPS sequence of Pseudomonas nitroreducens. In total, ten amino acid substitution mutants were successfully generated in this study. Our study showed that all mutants except FY47M-7 (G292S) showed sensitivity to 8.768% glyphosate. Multiple amino acid substitutions in FY43M-4 (R21C, N265S, and A329T) and FY47M-9 (L35F and A242V) completely abolished the glyphosate tolerance of Pseudomonas nitroreducens EPSPS. Therefore, further study should be carried out to identify the important mutation site that caused the change in glyphosate tolerance. Single site mutation in FY43M-8 (P71L) and FY47M-2 (T258A) showed sensitivity to 8.768% glyphosate, indicating that P71 and T258 are the important sites that enhance the glyphosate tolerance of Pseudomonas nitroreducens EPSPS. In our study, the glyphosate tolerance in FY47M-7 (G292S) mutant was not affected by the single mutation, whereas in FY47M-6 mutant with double mutations (L184F and G292C) showed sensitivity to 8.768% of glyphosate. This indicates that the glyphosate tolerance in the FY47M-6 mutant is affected by mutation at the L184 site, rather than at the G292 site of P. nitroreducens EPSPS.
Multiple-site mutations were generated on the EPSPS gene from Roseateles aquatilis (T38L, A40V, A222G, S224V, I225V, and G226L), whereby the cells expressing these mutants grew worse than wild- type in 150 mM glyphosate, indicating that these amino acid changes have reduced the glyphosate tolerance (Peng et al. 2012). On the other hand, a mutant consisting multiple mutation sites (G37V, D67N, T277S, D351G and R422G) was detected on Liriope spicata EPSPS through error-prone PCR, and it confers improved glyphosate tolerance up to 100 mM (Mao et al. 2017). Several mutations (N267S, P318R, and M425T) were observed on Ochrobactrum anthropic EPSPS after a few rounds of DNA shuffling and the mutant was able to restore its growth at 300 mM of glyphosate (Tian et al. 2011).
EPSPS gene mutants with single amino acid substitution which showed improved glyphosate tolerance have been reported by various groups (He et al. 2003; Zhou et al. 2006; Liang et al. 2008). The EPSPS gene from Oryza sativa was mutagenized through error-prone PCR causing a change of P106L in protein sequence. E.coli expressing P106L mutant showed threefold increase in glyphosate resistance and its affinity for glyphosate was decreased about 4.6-fold as compared to wild-type E.coli (Zhou et al. 2006). Cells expressing EPSPS mutant (T42M) from E. coli were able to grow well in 30 and 60 mM glyphosate, but the wild type grew poorly in the presence of glyphosate (He et al. 2003). The single amino acid substitution, A130S from Pseudomonas stutzeri A1501, showed 2.5-fold increase in glyphosate resistance (Liang et al. 2008). The amino acid changes obtained from our studies are novel, as they are all different as compared to those found in previous studies. In addition to our knowledge, no EPSPS gene source extracted from Pseudomonas nitroreducens was reported in previous studies.
Conclusion
We report in this study two strains of glyphosate-tolerant Pseudomonas species (FY43 and FY47) isolated from the soil samples obtained from oil palm plantation. Both strains displayed tolerance towards 8.768% (518.4 mM) of glyphosate, which is the highest among other reported Pseudomonas species. Ten amino acid substitution mutants were generated using error-prone PCR method. Multisite mutations found in FY43M-4 (R21C, N265S, A329T) and FY47M-9 (L35F, A242V) mutants and single site mutation in FY43M-8 (P71L) and FY47M-2 (T258A) mutants abolished the tolerance of P. nitroreducens EPSPS to 8.768% (518.4 mM) of glyphosate. FY47M-6 (L184F, G292C) showed reduced glyphosate tolerance to 8.768% (518.4 mM) of glyphosate, whereas glyphosate tolerance in FY47M-7 (G292S) mutant was maintained.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by the University of Malaya Grant, BKP078-2016 and RU006-2017.
Compliance with ethical standards
Conflict of interest
All the authors declare no conflict of interest.
References
- Aristilde L, Reed ML, Wilkes RA, et al. Glyphosate-induced specific and widespread perturbations in the metabolome of soil Pseudomonas species. Front Environ Sci. 2017;5:34. doi: 10.3389/fenvs.2017.00034. [DOI] [Google Scholar]
- Baerson SR, Rodriguez DJ, Tran M, et al. Glyphosate-resistant goosegrass. Identification of a mutation in the target enzyme 5-enolpyruvylshikimate-3-phosphate synthase. Plant Physiol. 2002;129:1265–1275. doi: 10.1104/pp.001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel B. Auxin biosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:51–66. doi: 10.1146/annurev.arplant.48.1.51. [DOI] [PubMed] [Google Scholar]
- Benslama O, Boulahrouf A. Isolation and characterization of glyphosate-degrading bacteria from different soils of Algeria. Afr J Microbiol Res. 2013;7:5587–5595. doi: 10.5897/AJMR2013.6080. [DOI] [Google Scholar]
- Eschenburg S, Healy ML, Priestman MA, et al. How the mutation glycine96 to alanine confers glyphosate insensitivity to 5-enolpyruvyl shikimate-3-phosphate synthase from Escherichia coli. Planta. 2002;216:129–135. doi: 10.1007/s00425-002-0908-0. [DOI] [PubMed] [Google Scholar]
- Fitzgibbon JE, Braymer HD. Cloning of a gene from Pseudomonas sp. strain PG2982 conferring increased glyphosate resistance. Appl Environ Microbiol. 1990;56:3382–3388. doi: 10.1128/AEM.56.11.3382-3388.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froger A, Hall JE. Transformation of plasmid DNA into E coli using the heat shock method. J Vis Exp. 2007;6:253. doi: 10.3791/253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke T, Healy-Fried ML, Han H, et al. Differential inhibition of class I and class II 5-enolpyruvylshikimate-3-phosphate synthases by tetrahedral reaction intermediate analogues. Biochemistry. 2007;46:13344–13351. doi: 10.1021/bi701095u. [DOI] [PubMed] [Google Scholar]
- Gonzini LC, Hart SE, Wax LM. Herbicide combinations for weed management in glyphosate-resistant soybean (Glycine max) Weed Technol. 1999;13(2):354–360. doi: 10.1017/S0890037X00041853. [DOI] [Google Scholar]
- He M, Nie YF, Xu P. A T42M substitution in bacterial 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) generates enzymes with increased resistance to glyphosate. Biosci Biotechnol Biochem. 2003;67:1405–1409. doi: 10.1271/bbb.67.1405. [DOI] [PubMed] [Google Scholar]
- Jacob GS, Garbow JR, Hallas LE, et al. Metabolism of glyphosate in Pseudomonas sp. strain LBr. Appl Environ Microbiol. 1988;54:2953–2958. doi: 10.1128/AEM.54.12.2953-2958.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryuchkova YV, Burygin GL, Gogoleva NE, et al. Isolation and characterization of a glyphosate-degrading rhizosphere strain, Enterobacter cloacae K7. Microbiol Res. 2013;169:99–105. doi: 10.1016/j.micres.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Kuklinsky-Sobral J, Araújo WL, Mendes R, et al. Isolation and characterization of endophytic bacteria from soybean (Glycine max) grown in soil treated with glyphosate herbicide. Plant Soil. 2005;273:91–99. doi: 10.1007/s11104-004-6894-1. [DOI] [Google Scholar]
- Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang A, Sha J, Lu W, et al. A single residue mutation of 5-enolpyruvylshikimate-3-phosphate synthase in Pseudomonas stutzeri enhances resistance to the herbicide glyphosate. Biotech Lett. 2008;30:1397–1401. doi: 10.1007/s10529-008-9703-8. [DOI] [PubMed] [Google Scholar]
- Ling LL, Keohavong P, Dias C, et al. Optimization of the polymerase chain reaction with regard to fidelity: modified T7, Taq, and vent DNA polymerases. PCR Methods Appl. 1991;1:63–69. doi: 10.1101/gr.1.1.63. [DOI] [PubMed] [Google Scholar]
- Looi FY (2016) Isolation and characterization of glyphosate degrading bacteria from oil palm plantation soil sample. Bachelor theses, Taylor’s University, Subang Jaya
- Manogaran M, Shukor MY, Yasid NA. Isolation and characterization of glyphosate-degrading bacteria isolated from local soils in Malaysia. Rend Fis Acc Lincei. 2017;28:471–479. doi: 10.1007/s12210-017-0620-4. [DOI] [Google Scholar]
- Mao C, Xie H, Chen S, et al. Error-prone PCR mutation of Ls-EPSPS gene from Liriope spicata conferring to its enhanced glyphosate-resistance. Pestic Biochem Physiol. 2017;141:90–95. doi: 10.1016/j.pestbp.2016.12.004. [DOI] [PubMed] [Google Scholar]
- Moneke A, Okpala G, Anyanwu C. Biodegradation of glyphosate herbicide in vitro using bacterial isolates from four rice fields. Afr J Biotech. 2010;9:4067–4074. [Google Scholar]
- Peng RH, Tian YS, Xiong AS, et al. A novel 5-enolpyruvylshikimate-3-phosphate synthase from Rahnella aquatilis with significantly reduced glyphosate sensitivity. PLoS ONE. 2012;7:e39579. doi: 10.1371/journal.pone.0039579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollegioni L, Schonbrunn E, Siehl D. Molecular basis of glyphosate resistance-different approaches through protein engineering. FEBS J. 2011;278:2753–2766. doi: 10.1111/j.1742-4658.2011.08214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata AA, Schrödl W, Aldin AA, et al. The effect of glyphosate on potential pathogens and beneficial members of poultry microbiota in vitro. Curr Microbiol. 2013;66:350–358. doi: 10.1007/s00284-012-0277-2. [DOI] [PubMed] [Google Scholar]
- Stalker DM, Hiatt WR, Comai L. A single amino acid substitution in the enzyme 5-enolpyruvylshikimate-3-phosphate synthase confers resistance to the herbicide glyphosate. J Biol Chem. 1985;260:4724–4728. [PubMed] [Google Scholar]
- Tian YS, Xu J, Xiong AS, et al. Improvement of glyphosate resistance through concurrent mutations in three amino acids of the Ochrobactrum 5-Enopyruvylshikimate-3-phosphate synthase. Appl Environ Microbiol. 2011;77:8409–8414. doi: 10.1128/AEM.05271-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom Hall Ibis Biosciences (1997) BioEdit (version 7.2.5). https://www.mbio.ncsu.edu/bioedit/bioedit.html. Accessed 25 March 2019
- Widdel F. Theory and measurement of bacterial growth. Bremen: Grundpraktikum Mikrobiologie, Universität Bremen; 2010. [Google Scholar]
- Zhou M, Xu H, Wei X, et al. Identification of a glyphosate-resistant mutant of rice 5-enolpyruvylshikimate 3-phosphate synthase using a directed evolution strategy. Plant Physiol. 2006;140:184–195. doi: 10.1104/pp.105.068577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.