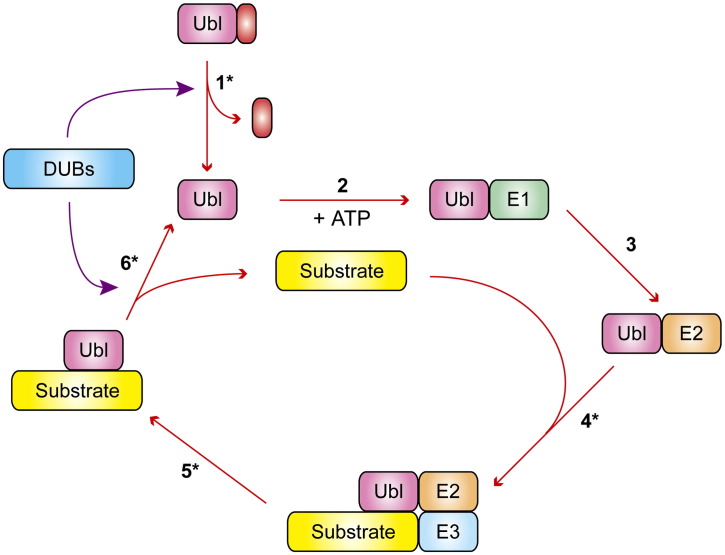
Fig. 1.
General schematic for Ubl conjugation and deconjugation and points of viral interference. Proteolytic maturation of Ubl (including Ub) precursor proteins by DUBs (step 1) exposes a new C-terminus in the modifier, which is then activated by E1 in an ATP-dependent manner (step 2) and next transferred to the E2 (step 3). An E3 generally recognizes the target protein (step 4) and facilitates the ligation of the Ubl to it (step 5), altering its protein interaction repertory and thereby its function. Polyubiquitination as a particular example of Ubl modification of a protein can lead to its proteasomal degradation. Ubl deconjugation by DUBs reversely regulates target protein function and, additionally, replenishes the pool of free Ubl (step 6). Points of viral interference are marked (*). Viruses are known to intervene with the Ub conjugation pathway (at steps 4 and 5, see text), but also target Ubl deconjugation (step 6), inclusively referred to as deubiquitination, which is the principal theme of this review. Viruses may also interfere with Ubl precursor processing (step 1), as suggested by the ability of some viral DUBs to process the ISG15 precursor protein (see text).