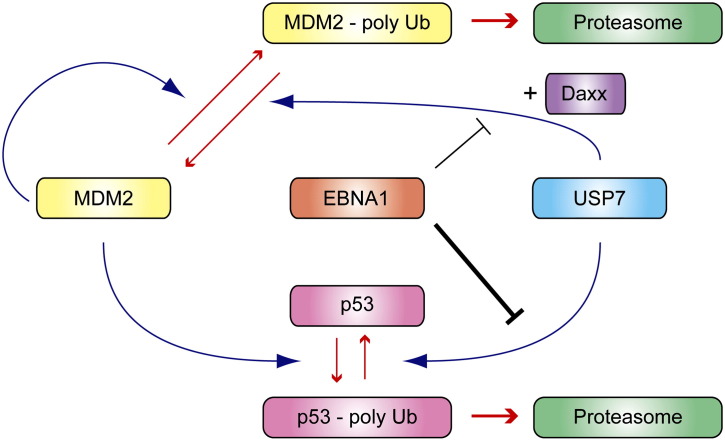
Fig. 2.
Model for regulation of the p53-MDM2 pathway by USP7 and interference by EBNA1. The E3 enzyme MDM2 catalyses both autoubiquitination and ubiquitination of p53 leading to proteasomal degradation in both cases. Deubiquitination by USP7 stabilizes p53 and MDM2, with the adaptor protein Daxx directing USP7 to MDM2. Partial reduction of USP7 activity by RNAi destabilizes p53 through reduced deubiquitination. Contrarily, genetic knockout of USP7 increasingly commits MDM2 to proteasomal degradation thus leading to an important reduction in p53 ubiquitination and, indirectly, effective p53 stabilization. The EBV protein EBNA1, MDM2, and p53 compete for same binding site on USP7, with affinities decreasing in this order. Hence, inhibition of USP7 by EBNA1 may exhibit selectivity and contribute twofold to p53 destabilization. By blocking the deubiquitination of p53 more efficiently than the deubiquitination of MDM2, it may allow for sufficient levels of MDM2 that maintain p53 ubiquitination.