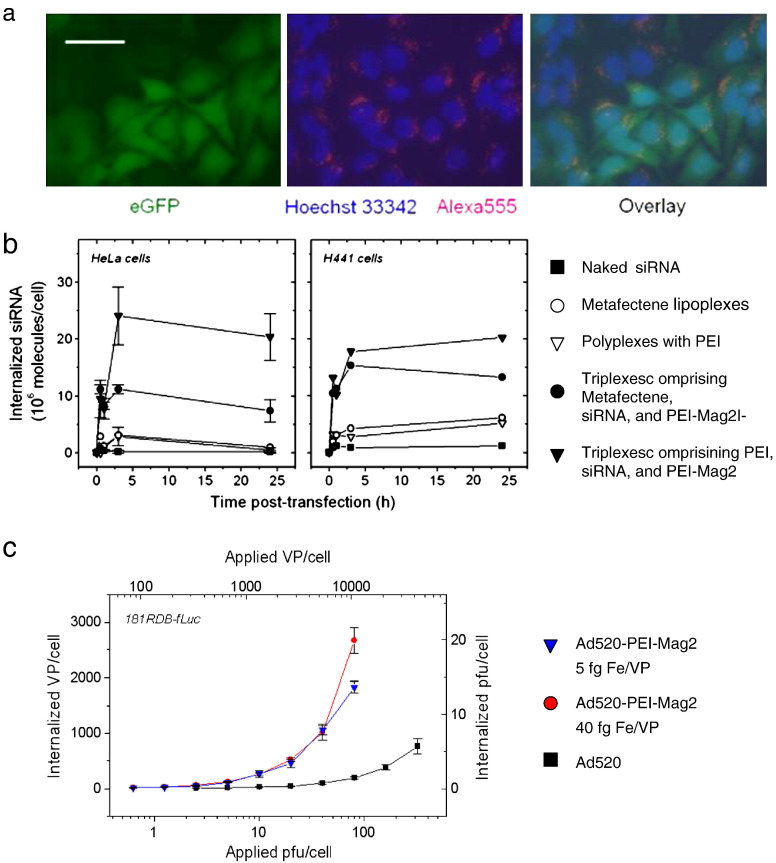
Fig. 9.
Internalization of magnetic vectors. (a) HeLa-GFP cells were incubated for 30 min at the magnetic plate with PEI-Mag2/PEI/GFP-siRNA-Alexa555 triplexes at siRNA concentration of 100 ng/10,000 cells/0.33 cm2; iron-to-siRNA wt/wt ratio of 0.5, PEI/siRNA ratio of N/P = 10 and observed after 48 h with a fluorescence microscope. Bar = 50 μm. Hoechst 33342 was used as a nuclear counterstain. The pictures show fluorescence images taken at 490/509 nm (green fluorescence) for eGFP fluorescence, 510/650 nm (red fluorescence) for GFP-siRNA-Alexa555 and at 350/461 nm (blue fluorescence) for Hoechst 33342 nuclear staining, or overlays thereof. Fluorescence microscopy data prove the association of the magnetic transfection complexes with a majority of the cells and are indicative of internalization into cells. Fluorescently labeled siRNA triplexes comprising magnetic nanoparticles appear to be localized predominantly around the nuclei. (b) Vector internalization in HeLa human cervical epithelial adenocarcinoma cells and H441 human lung epithelial cells. The cells were transfected in a 96-well plate using 125I-labeled siRNA complexes. The siRNA dose was 100 ng per well. At time points 0.5 h, 1 h, 3 h, and 24 h post-transfection the cells were incubated with heparin solution in the presence of sodium azide to remove extracellularly bound complexes, washed, trypsinized, and collected. Cell-associated radioactivity was measured with a gamma counter. The applied dose of the radioactively labeled siRNA complexes was used as a reference. The results were recalculated in terms of the siRNA molecules internalized per seeded cell. (c) Oncolytic adenovirus Ad520 uptake in multidrug resistant 181RDB-fLuc cells as a function of the applied virus dose. 181RDB-fLuc cells were infected with 125I-labeled Ad520 or its magnetic virus complexes with PEI-Mag2 nanoparticles in 7.5% FCS-containing cell culture medium for 30 min. The complexes were prepared at ratios of 5 and 40 fg Fe/VP in OptiMEM. Six hours post-infection, the infected cells were washed with PBS, incubated with heparin solution and then lysed in lysis buffer. Cell-associated radioactivity was measured in the cell lysate using a gamma counter.
Panel (a) was reproduced with permission from Thomson Reuters (Scientific) Ltd: Curr Opin Mol Ther. [85]; panel (b) was reproduced with permission from Humana Press: Meth Mol Biol [76]; and panel (c) was reproduced with permission from ACS Publications: Molecular pharmaceutics [55].