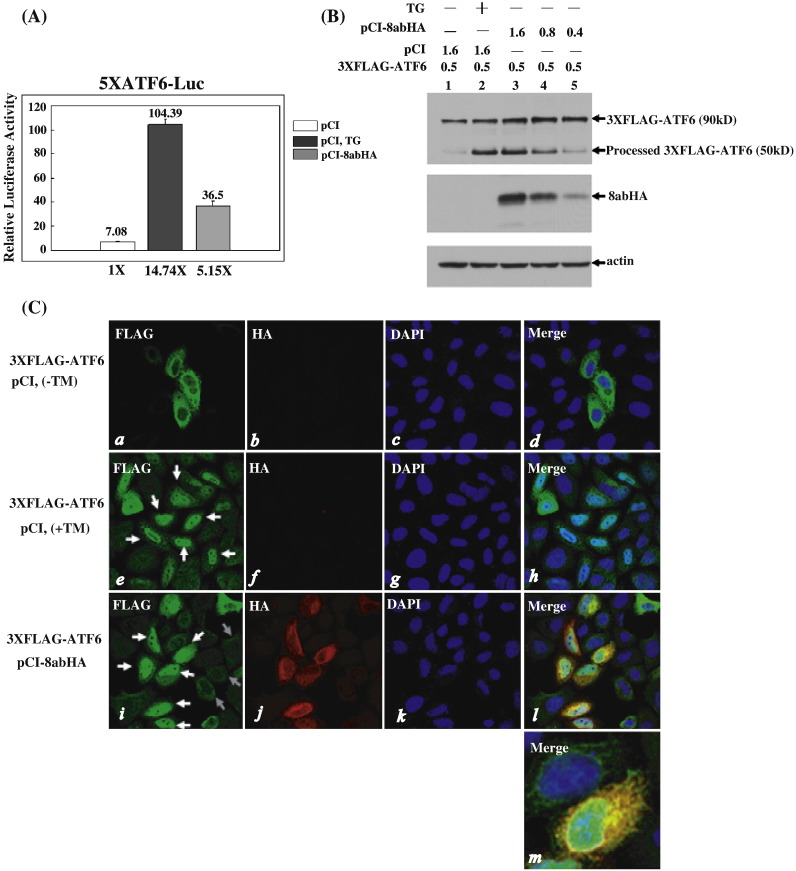
Fig. 5.
Activation of ATF6 by 8ab. (A) 8ab protein induces the ATF6-dependent transcriptional activity. HeLa cells were transiently co-transfected with pCI8abHA and the reporter plasmid p5XATF6-GL3 for 40 h (gray bar). Control cells were co-transfected with reporter plasmids and empty pCI vector for 24 h, and then induced with or without TG 500 nM for an additional 16 h (black or white bar). In each case, pRL-TK encoding Renilla luciferase was co-transfected as an internal control. The cell lysates were harvested for dual luciferase assay as described. (B) Immunoblotting analysis of the proteolysis of ATF6. HeLa cells were co-transfected with 0.5 μg of 3XFLAG-ATF6 and different amounts of pCI-8abHA as indicated (lanes 3–5). After 30 h, cell lysates were harvested for Western blot analysis with anti-FLAG and anti-HA antibodies. Control cells were co-transfected with 0.5 μg of 3XFLAG-ATF6 and empty vector for 24 h, and then treated or untreated with TG 500 nM for an additional 6 h (lane 2 and lane1). The full-length 3XFLAG-ATF6 (90kD) and the processed N-terminal domain of 3XFLAG-ATF6 (50 kD) are indicated. (C) Indirect immunofluorescence analysis of the nuclear translocation of 3XFLAG-ATF6. HeLa cells were transiently co-transfected with 3XFLAG-ATF6 and pCI8abHA for 30 h (panels i–l), or transfected with 3XFLAG-ATF6 and empty vector for 20 h, and then treated (panels e–h) or untreated with 5 μg/ml of tunicamycin for an additional 4 h (panels a–d). After being fixed and permeabilized, cells were labeled with anti-HA and anti-FLAG antibodies. Cells were co-stained with DAPI to reveal the nucleus. In panels e and i, white arrows point to the cells exhibiting nuclear translocation of processed 3XFLAG-ATF6, and gray arrows point to cells in which ATF6 exhibits the ER distribution pattern. At higher magnification (panel m), partial co-localization of ATF6 and 8ab in the ER is revealed.