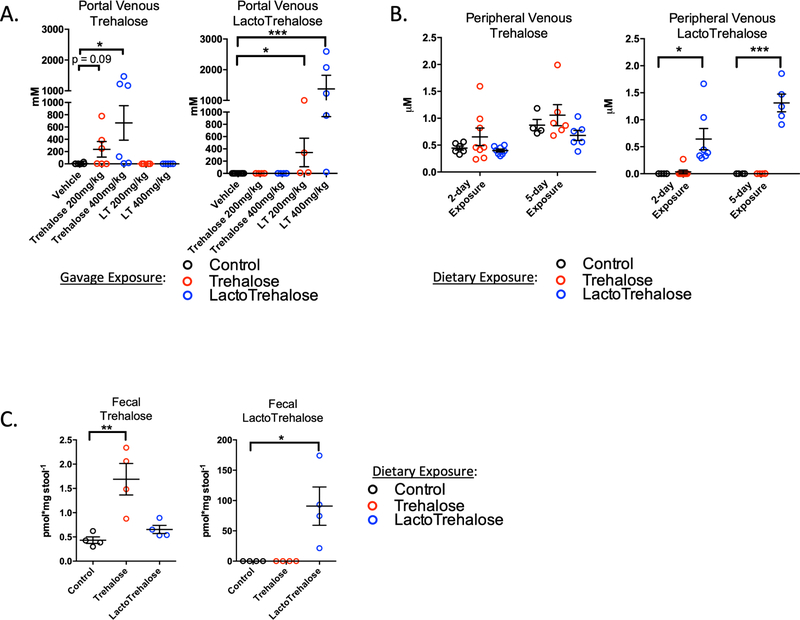
Figure 4. Rapid oral bioavailability of trehalose and lactotrehalose in the hepatic portal circulation.
A. Gas Chromatography and Mass Spectrometry (GC-MS) quantification of acute portal venous [trehalose] (left) or [lactotrehalose] (right) 15’ after oral gavage of either sterile water or 200–400mg/kg trehalose or lactotrehalose. B. GC-MS quantification of steady-state peripheral [trehalose] or [lactotrehalose] after 2- and 5-day exposure to trehalose (red dots) or lactotrehalose (blue dots) (3% in water ad libitum). C. GC-MS quantification of steady-state fecal [trehalose] or [lactotrehalose] after 5-day exposure to trehalose (red dots) or lactotrehalose (blue dots) (3% in water ad libitum, 5 days). *, ***, P < 0.05, 0.001 versus comparison group by two-tailed t-test with Bonferroni-Dunn post hoc correction for multiple comparisons.