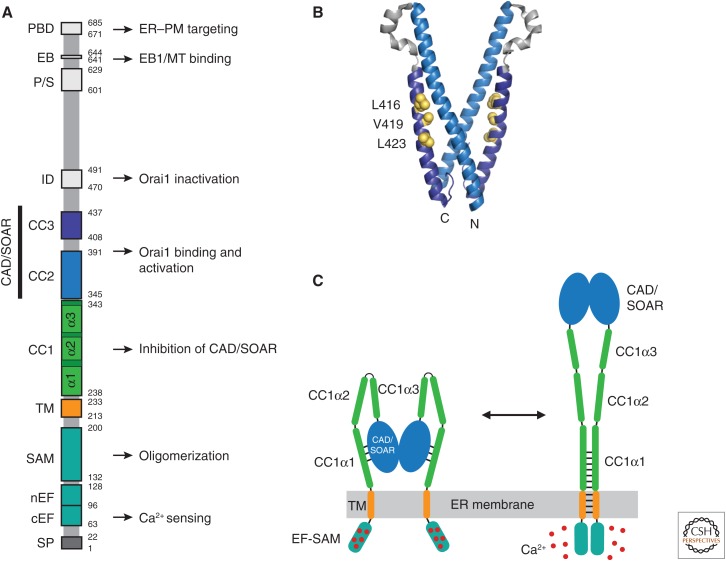
Figure 2.
STIM1 functional domains and a model for activation. (A) Domain organization of STIM1. Functional domains include the signal peptide (SP), canonical EF-hand (cEF), noncanonical EF-hand (nEF), sterile α motif (SAM), transmembrane (TM) domain, putative coiled-coil domains 1–3 (CC1–3), Ca2+ release-activated Ca2+ (CRAC) activation domain (CAD, aa 342–448) or STIM-Orai-activating region (SOAR, aa 344–442), inactivation domain (ID), proline/serine-rich domain (P/S), EB1-binding (EB) domain, and the polybasic domain (PBD). Known functions are indicated. (B) Crystal structure of the dimeric CAD/SOAR (3TEQ.pdb) (Yang et al. 2012). Amino- and carboxy-terminal ends of the front subunit and selected residues implicated in interactions with CC1 (L416, V419, L423) are indicated in yellow. (C) A coiled-coil model for STIM1 activation by ER Ca2+ depletion (regions downstream from CAD/SOAR omitted for clarity). In the resting state (left), the luminal EF-SAM domains are bound to 5–6 Ca2+ ions and separated, which allows CC1α1 to bind to CAD/SOAR and sequester it close to the endoplasmic reticulum (ER) membrane. Following store depletion (right), Ca2+ release triggers dimerization of the EF-SAM domain, bringing the TM domains together to form a coiled-coil. This rearrangement favors dissociation of CC1α1 from CAD/SOAR and extends the coiled-coil beyond the ER membrane to move CAD/SOAR toward the plasma membrane (PM).