Figure 5.
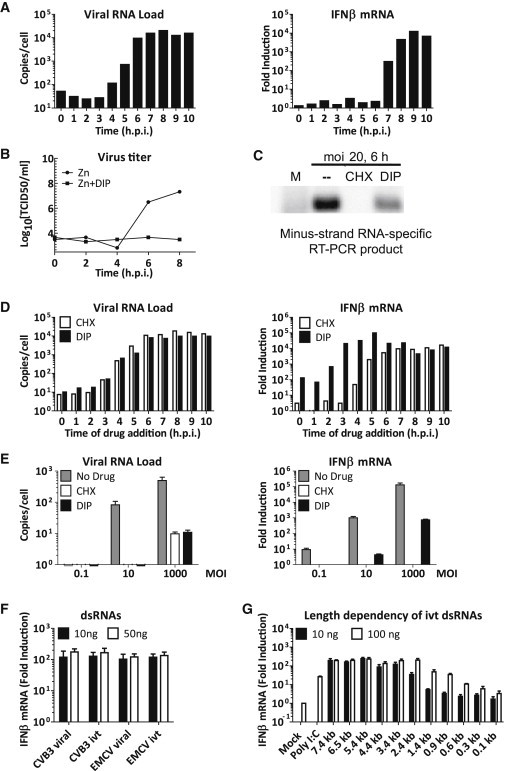
Viral RNA Recognition in Infected Cells and Characterization of Determinant for MDA5 Activation in RF
(A) HeLa cells were infected with mengo-Zn (MOI 10) and samples were taken every hour postinfection. Total RNA was extracted and viral RNA and host IFN-β mRNA levels were assayed by real-time qPCR.
(B) Cells were infected with mengo-Zn in the absence or presence of DIP (100 μM). Production of infectious particles was determined by end-point titration on BHK-21 cells.
(C) HeLa cells were mock-infected or infected with mengo-Zn (MOI 20) in the absence or presence of CHX (10 μg/ml) or DIP (100 μM) and total RNA was extracted at 6 hr.p.i. dsRNA fraction was purified by LiCl precipitation and subjected to RT-PCR using primers specific to viral (−) RNA.
(D) HeLa cells were infected with mengo-Zn at MOI 10. CHX (10 μg/ml) or DIP (100 μM) was added to infected cells either at time of infection or at indicated times postinfection. All samples were harvested at 12 hr.p.i. Total RNA was extracted and viral RNA and host IFN-β mRNA levels were analyzed by real-time qPCR.
(E) HeLa cells were infected with mengo-Zn at indicated MOI’s in the absence or presence of CHX (10 μg/ml) or DIP (100 μM). Total RNA was extracted at 10 hr.p.i. and viral RNA and host IFN-β mRNA levels were measured by real-time qPCR. Data presented as mean ± SD.
(F) RFs of CVB3 and mengovirus as well as in vitro transcribed dsRNAs of viral sequences were transfected into RIG-I−/− MEFs in the presence of CHX (10 μg/ml) at indicated amounts per well in 24-well format. IFN-β mRNA levels were determined at 8 hr.p.t. by real-time qPCR. Data presented as mean ± SD.
(G) polyIC or in vitro transcribed dsRNAs of indicated length were transfected into RIG-I−/− MEFs in the presence of CHX (10 μg/ml) at indicated amounts. IFN-β mRNA levels were determined at 8 hr.p.t. by real-time qPCR. Data presented as mean ± SD.
See also Figures S4 and S5.
