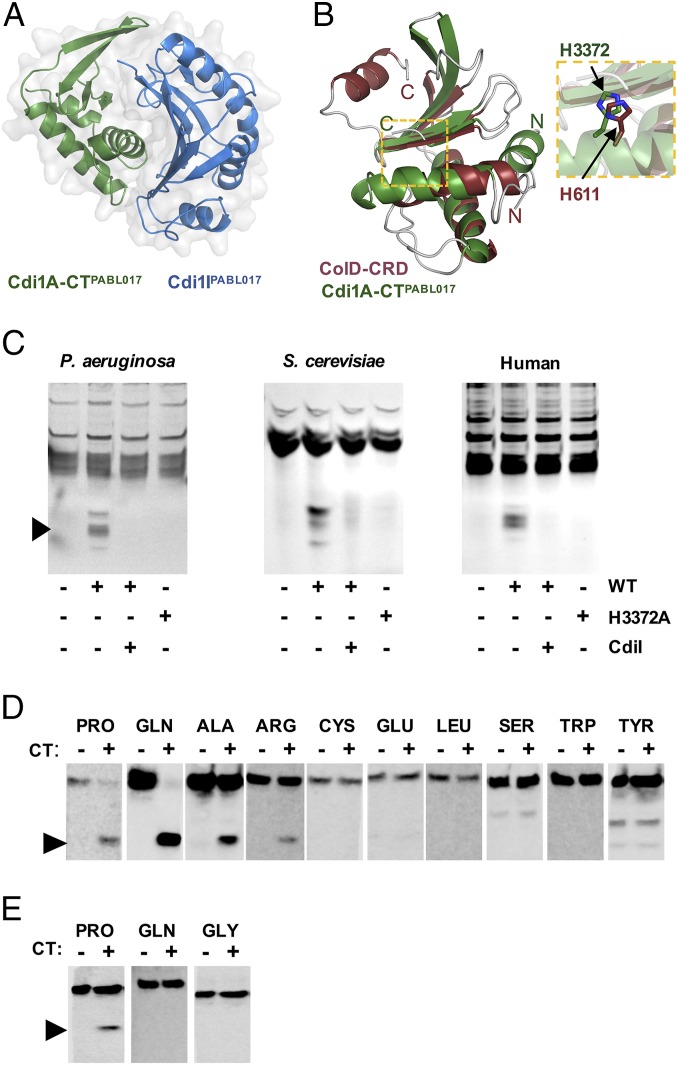
Fig. 4.
Cdi1A-CTPABL017 is a tRNase. (A) The crystal structure of recombinant Cdi1A-CTPABL017 was solved in complex with recombinant Cdi1IPABL017. (B) Cdi1A-CTPABL017 structure is highly similar to the ColD-CRD. The individual structures are overlaid to reveal structural similarity and highlight the position and orientation of the catalytic His611 from ColD with the putative catalytic His3372 of Cdi1APABL017 (Inset). (C) Purified Cdi1A-CTPABL017 (WT) or Cdi1A-CT[H3372A]PABL017 (H3372A) was incubated with tRNA preparations from Saccharomyces cerevisiae (Sigma) or total nucleic extracts from P. aeruginosa or HeLa cells at 37 °C for 60 min. The preparations were separated on a denaturing polyacrylamide gel, stained with ethidium bromide and visualized with UV light. Cdi1A-CTPABL017 protein was preincubated with purified Cdi1IPABL017 protein for 30 min where indicated (CdiI). Black arrowhead denotes cleaved products. Northern blot analysis of (D) P. aeruginosa or (E) HeLa cell total nucleic acid preparations treated with WT Cdi1A-CTPABL017 as above and hybridized with tRNA-specific probes. Black arrowheads indicate cleaved tRNA molecules.