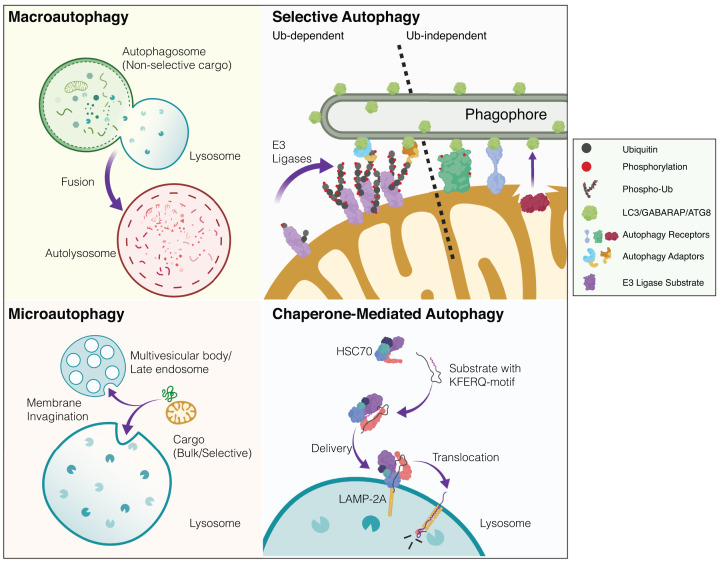
Figure 1. Simplified overview of mammalian autophagy modalities.
There are several distinct modes of autophagy in eukaryotic cells. These subtypes are defined according to how cargo is recognised and delivered to the acidic or degradative cellular subcompartments (i.e. lysosomes or late endosomes). Macroautophagy: cytoplasmic constituents such as organelles and protein aggregates are non-selectively engulfed in a double-membrane bound autophagosome. This bulk sequestration of cellular matter results in degradation upon the formation of the autolysosome (autophagosome-lysosome fusion). The homoeostatic balance between degradation and biosynthesis is ensured by the process of autophagosome-lysosome reformation (not depicted). Microautophagy: in contrast with autophagosomal delivery, cargo destined for destruction enters the endolysosomal system via membrane invagination. This occurs in non-selective and selective manners with chaperones. Multi-vesicular bodies (MVBs) are also known to participate in microautophagy. Selective autophagy: distinct substrates (organelles, proteinaceous/membranous aggregates, pathogens) are recognised via priming by cargo-specific receptors. Selective autophagy of organelles (organellophagy) can occur in a ubiquitin-dependent or -independent fashion. The depicted example shows mitophagy where in response to stress, ubiquitin E3 ligases can trigger mitochondrial ubiquitylation, thereby recruiting specific adaptor proteins that engage the autophagy machinery. In ubiquitin-independent selective autophagy, specific receptors directly engage LC3/GABARAP/ATG8 proteins to drive the encapsulation and elimination of the organelle. Lipids such as cardiolipin and ceramide have also been reported as mitophagy receptors (not depicted). Although mitophagy is largely studied as an intracellular process, transcellular mitophagy (known as axonal transmitophagy in neurons) has been described in the CNS (not depicted). Mitochondrial subdomains can also be excised and delivered to the lysosome via mitochondrial-derived vesicles (MDVs, not shown). Chaperone-mediated autophagy (CMA): substrate proteins containing a distinct motif are selectively recognised by chaperones and delivered to lysosomes where they undergo translocation and destruction. Created with BioRender.