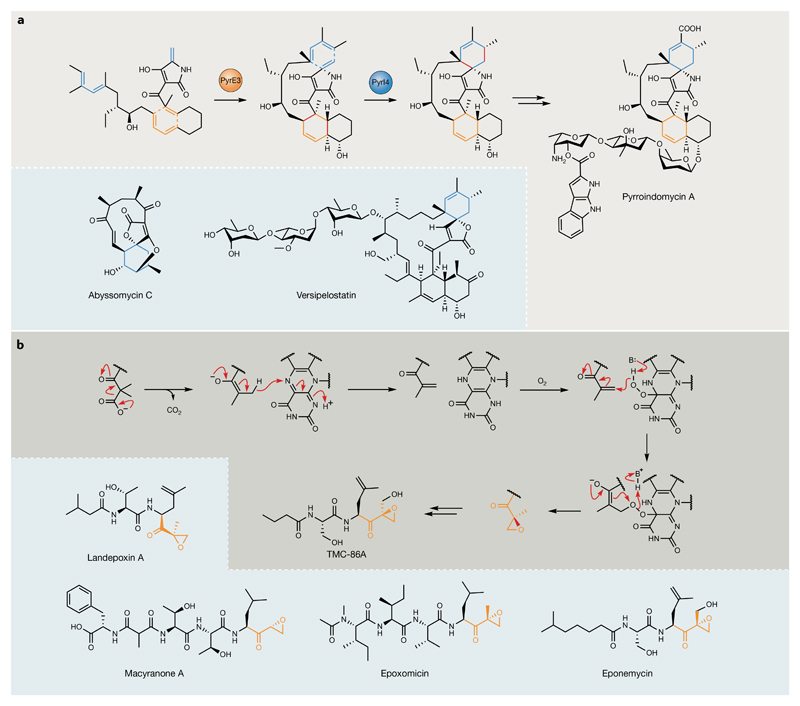
Fig. 4. Unusual post-polyketide synthase/nonribosomal peptide synthetase enzymology.
a | Enzymatic [4+2] cycloadditions during pyrroindomycin biosynthesis. Grey panel: Diels–Alder-type cyclizations catalysed by PyrE3 (orange) and PyrI4 (blue)56. New bonds formed are highlighted in red. Blue panel: six-membered rings (highlighted in blue) installed by PyrI4 homologues during abyssomycin57 and versipelostatin55 biosynthesis. b | Grey panel: TmcF catalyses a remarkable decarboxylation–dehydrogenation–oxygenation transformation to generate the epoxyketone moiety present in TMC-86A62. The new bond formed to close the epoxide ring is highlighted in red. Blue panel: select examples of natural product proteasome inhibitors that are proposed to employ TmcF homologues during their biosynthesis to install α/β-epoxyketone warhead moieties59–62. Epoxyketone moieties introduced by TmcF and its homologues are highlighted in orange.