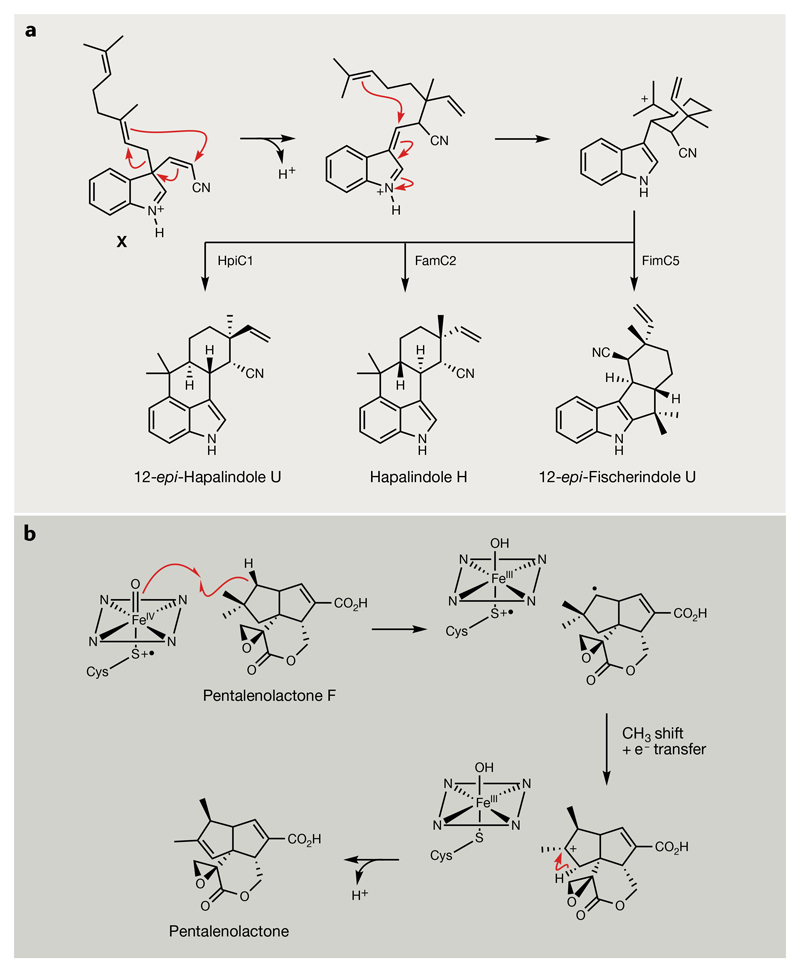
Fig. 7. Unusual transformations from terpene biosynthesis.
a | Cyclization of the common 3-geranyl-3-isocyanylvinyl indolenine intermediate (X) catalysed by Stig cyclases. The remarkable transformation catalysed involves a rare Cope-like rearrangement, a 6-exo-trig cyclization and an electrophilic aromatic substitution102–105. Different Stig cyclases possess specific stereoselectivity and regioselectivity, as illustrated by the different products generated by HpiC1, FamC2 and FimC5. b | Proposed mechanism of oxidative rearrangement in the conversion of pentalenolactone F into pentalenolactone by the cytochrome P450, PntF117,229. The mechanism of transient neopentyl cation intermediate formation remains to be experimentally verified.