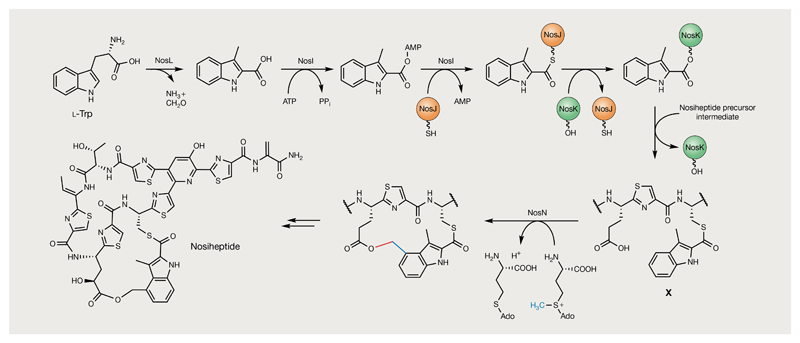
Fig. 9. Formation of the side-ring system during the biosynthesis of nosiheptide.
3-Methyl-2-indolic acid (MIA) is formed by the rearrangement of l-Trp catalysed by NosL and is subsequently introduced into the nosiheptide side-ring system by NosIJK147–154. These enzymes transfer the MIA moiety to an unmodified Cys residue in a linear pentathiazolyl nosiheptide intermediate. NosN is proposed to methylate MIA at the C4 position to generate a key methylene radical intermediate that is subsequently linked via an ester bond to Glu6 in the pentathiazolyl intermediate (X)155. The S-adenosyl methionine (SAM)-derived methyl group introduced by NosN is highlighted in blue and the bond formed with Glu6 to close the nosiheptide side-ring system is highlighted in red.