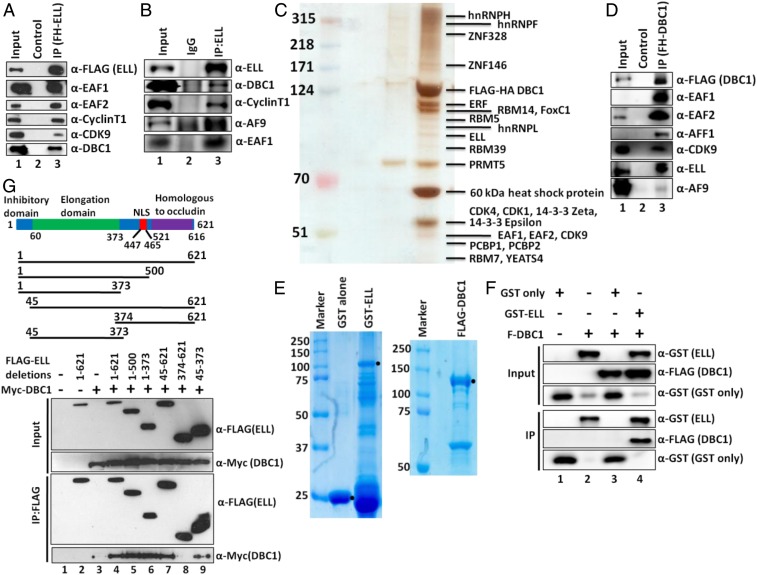
Fig. 1.
DBC1 and ELL interact both in vitro and in vivo within mammalian cells. (A and B) IP and Western blotting analysis showing identification of DBC1 as an interactor of (A) ectopic and (B) endogenous ELL along with other known interacting components (IgG: immunoglobulin G). (C) Purification of DBC1-associated protein complex from a stable cell line that ectopically expresses DBC1 as FLAG-HA−tagged. Eluted proteins were run on 4 to 12% gradient gel and silver-stained for their visualization. Individual bands were excised, and proteins were identified by mass spectrometric analysis. (D) IP and Western blotting analysis for identification of SEC component association with DBC1. (E) Coomassie staining of purified recombinant GST, GST–ELL (using bacterial expression system), and FLAG-DBC1 (using baculoviral expression system). GST–ELL shows significant degradation in our purification. (F) In vitro direct interaction assay with purified proteins showing direct interaction of DBC1 with GST–ELL but not GST alone. (G) Cartoon diagram showing domains of ELL that are important for regulating its functions (Upper) (NLS: nuclear localization signal). Depiction of ELL domains that have been used for its interaction assay with DBC1 by co-IP analysis (Middle). Western blot analysis showing interaction of indicated domains of ELL with DBC1 within mammalian cells by co-IP analysis (Lower).