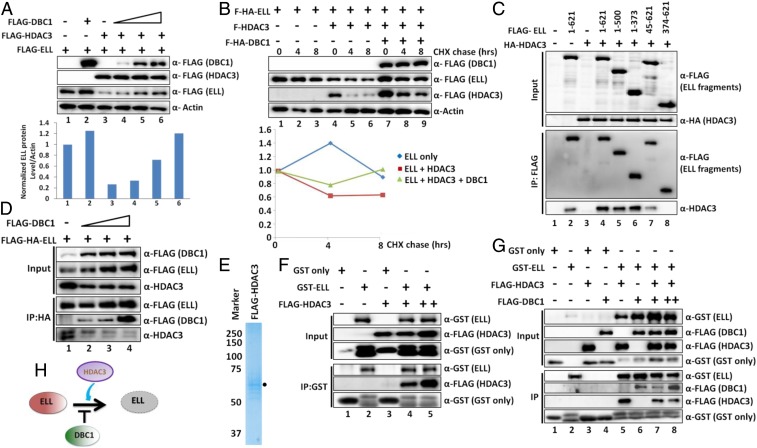
Fig. 3.
Competitive binding between DBC1 and HDAC3 to ELL. (A) Western blot analysis showing the rescue of HDAC3-mediated ELL degradation through concomitant overexpression of DBC1 (Upper) and quantitation of ELL protein relative to Actin (Lower). (B) CHX chase assay showing reduced degradation kinetics of ectopically expressed ELL mediated by HDAC3 in presence of DBC1 at different time points after addition of CHX in 293T cells (Upper) and quantitation of relative ELL degradation over time (Lower). (C) Western blot analysis showing the interaction of indicated ELL domains with HDAC3 within mammalian cells by co-IP analysis. (D) IP and Western blot analysis showing reduced interaction of HDAC3 with ELL upon increasing expression and binding of DBC1 to ELL within 293T cells. (E) Purification of HDAC3 through its overexpression in mammalian 293T cells. (F) In vitro direct interaction assay with purified proteins showing direct interaction of HDAC3 with GST–ELL but not GST alone. (G) In vitro direct binding assay, using purified proteins, showing competitive binding between HDAC3 and DBC1 to ELL. (H) Cartoon diagram depicting opposing roles of DBC1 and HDAC3 in regulation of ELL stability, wherein DBC1 stabilizes and HDAC3 destabilizes ELL protein.