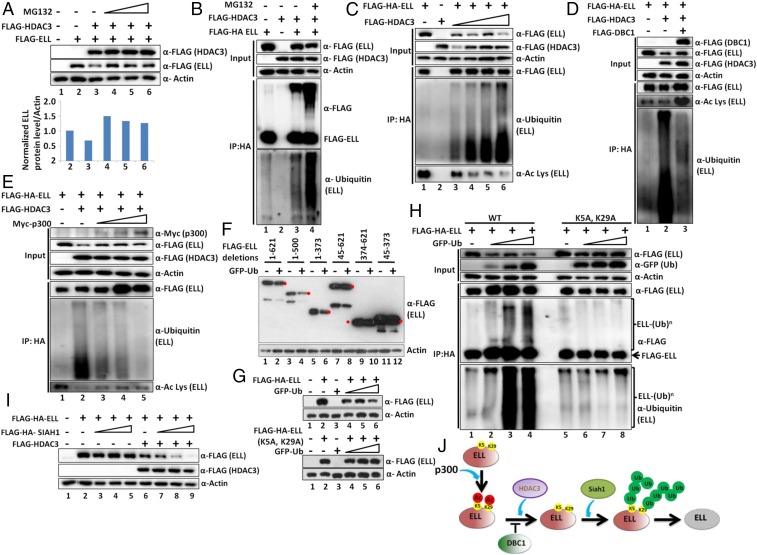
Fig. 5.
Poly-ubiquitylation of ELL at N-terminal K5 and K29 residues in presence of HDAC3 dictates ELL degradation by Siah1 within mammalian cells. (A) Western blot analysis showing rescue of HDAC3-mediated ELL degradation through addition of ubiquitin proteasome inhibitor MG132 (Upper) and quantitation of ELL level (Lower). (B) Western blot analysis showing increased polyubiquitylation of ELL by coexpression of HDAC3. The polyubiquitylation signal is further enhanced upon addition of MG132. (C) Western blot analysis showing a correlation of increased polyubiquitylation and decreased acetylation of ELL mediated by increased expression of HDAC3. (D and E) Western blot analysis showing strong correlation of increased ELL acetylation and reduced polyubiquitylation in presence of HDAC3 through concomitant overexpression of (D) DBC1 and (E) p300. (F) Western blot analysis showing the specific domain of ELL that shows sensitivity to ubiquitin-mediated degradation. Red filled circles indicate target protein bands, and others are either degradation or nonspecific proteins. (G) Western blot analysis showing sensitivity of ELL (WT) and ELL (K5A, K29A) mutant toward ubiquitin-mediated degradation. (H) Western blot analysis showing reduced polyubiquitylation of ELL (K5A, K29A) mutant in presence of overexpressed ubiquitin. (I) Western blot analysis showing increased ELL degradation by the ubiquitin E3 ligase Siah1 only in presence of HDAC3. (J) Cartoon diagram depicting the role of p300-mediated acetylation, DBC1-mediated acetylation protection, HDAC3-mediated deacetylation, and Siah1-mediated polyubiquitylation in overall regulation of ELL stability within mammalian cells.