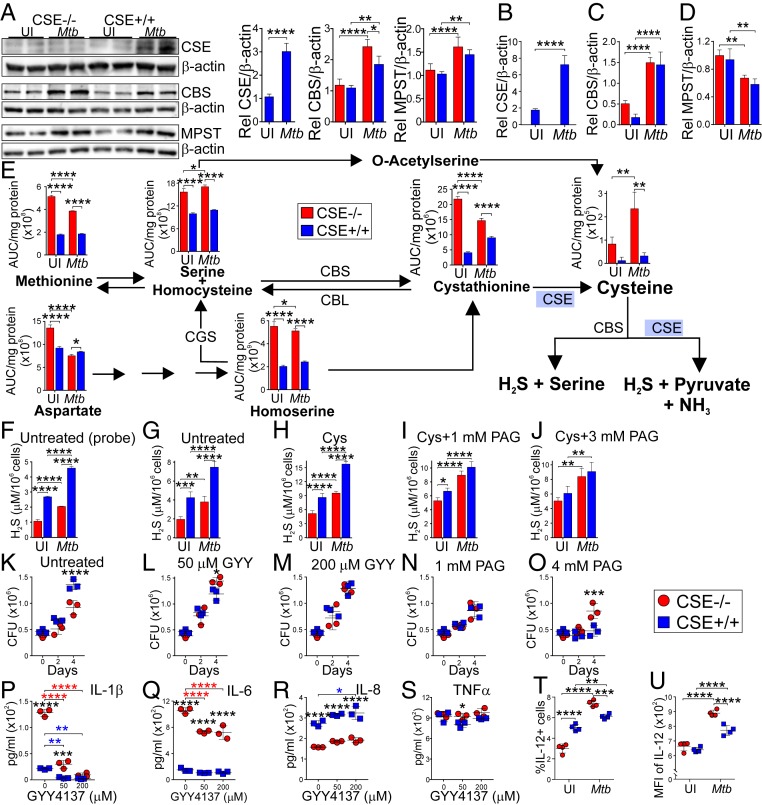
Fig. 4.
Endogenous H2S produced by CSE supports Mtb growth in macrophages. (A) Western blot showing expression of CSE, CBS, and MPST in uninfected and Mtb-infected peritoneal macrophages (PMs) at 24 h postinfection. Densitometric quantitation of the Western blot bands relative to the β-actin band intensities are shown in the bar graphs (Right). Expression in the uninfected WT peritoneal macrophages has been normalized to 1. Transcription of the (B) CSE, (C) CBS, and (D) MPST genes in uninfected and Mtb-infected PMs relative (Rel) to transcription of the β-actin gene at 24 h postinfection. (E) LC-MS/MS quantitation of amino acids involved in the endogenous H2S pathway in uninfected and Mtb-infected PMs at 24 h postinfection. (F–J) Measurement of H2S in the supernatants of uninfected and Mtb-infected PMs at 24 h postinfection using a probe-based H2S microsensor (F), and the conventional methylene blue method (G) of untreated and 4-h treatments with (H) 2 mM Cys, (I) 2 mM Cys and 1 mM PAG (an irreversible and specific inhibitor of CSE), (J) 2 mM Cys and 3 mM PAG. (K) Bacterial burden of Mtb-infected PMs at day 0, day 2, and day 4 postinfection, after treatment with (L) 50 µM GYY4137 (a slow releaser of H2S), (M) 200 µM GYY4137, (N) 1 mM PAG, and (O) 4 mM PAG. Error bars represent SD of the mean of four replicates. (P–S) Cytokine levels of (P) IL-1β, (Q) IL-6, (R) IL-8, and (S) TNF-α in the supernatants of Mtb-infected PMs at 24 h postinfection. Blue asterisks indicate significance between WT PMs; red asterisks indicate significance between CSE−/− PMs; black asterisks indicate significance between WT and CSE−/− PMs. (T–U) Intracellular measurements of IL-12 production of uninfected and Mtb-infected PMs at 24 h postinfection. Two-way ANOVA was used to determine statistical significance of all of the data. Error bars represent SD of the mean of three to four biological replicates. Data are representative of two independent experiments; ****P < 0.0001; ***P < 0.001; **P < 0.01; *P < 0.05.