Figure 4.
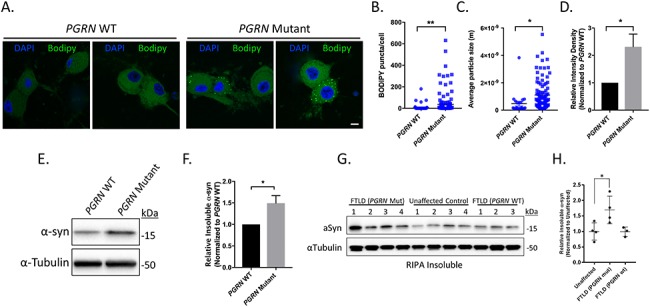
Lipid and insoluble α-synuclein accumulation in iPSC-derived PGRN mutant neurons and cortical tissue lysates from FTLD patients with PGRN mutations. (A) Neutral lipids in PGRN WT and mutant neurons were visualized using BODIPY 493/503 via IF (day 125 post-differentiation) (n = 3, 18–65 cells/experiment) Scale bar = 10 μm. Quantification of (B) BODIPY puncta/cell, (C) average BODIPY particle size, and (D) relative BODIPY intensity density in PGRN WT and mutant neurons. PGRN mutant neurons intensity samples were divided by the mean value obtained from PGRN WT samples. (E) Western blots of the insoluble protein fraction of PGRN WT and mutant neurons samples immunoblotted for α-synuclein and α-tubulin (loading control) (day 125 post-differentiation). (F) Quantification of insoluble α-synuclein in PGRN WT and mutant neuron lysates (n = 4). (G) Western blots of the insoluble protein fraction from cortical tissue samples from FTLD (PGRN mutant), unaffected control and FTLD (PGRN WT) immunoblotted for α-synuclein and α-tubulin (loading control). (H) Quantification of insoluble α-synuclein in cortical tissue samples. α-synuclein expression was normalized to the corresponding α-tubulin and divided by the mean value obtained from the unaffected samples. The data are presented as the mean ± SEM, *P < 0.05, **P < 0.01, (B,C,F) two tailed Student’s t-test, (H) one-way ANOVA followed by Tukeys multiple comparisons post hoc test.
