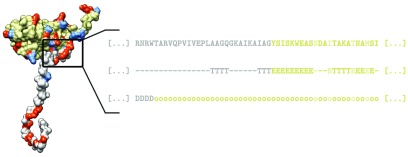
Figure 1.
Sketch of the capsid protein model. Experimentally determined structure of a viral CP is obtained from PDB/VIPERdb—in this case, chain C of the CP of BMV (PDB: 1JS9). Ionizable AA residues are shown in red and blue (positively and negatively charged, respectively), while the predicted split of the protein into the N-tail and the structured part of the CP, based on the assigned secondary structure, is shown in gray and beige, respectively. Inset shows the primary AA sequence, assigned secondary structure, and the prediction of intrinsic disorder in a region of the protein. Secondary structure is assigned to the AA residues in the CP using STRIDE, and we define the end of the N-tail as the first occurrence of a given structural element—in this case, a β-sheet (E). Alternatively, we split the protein into the N-tail and CP based on the predicted disordered regions (D) in it, where the tail ends at the first occurrence of an ordered region (o)—in this case, this results in a shorter predicted tail. Afterwards, we assign the ionizable residues in the protein a fractional charge, allowing us to obtain the charge on the tail and the CP at any pH. Residues which are predicted to be buried (not exposed to the solvent) are not considered as ionizable, and are highlighted in a lighter color in the primary sequence.