Figure 5.
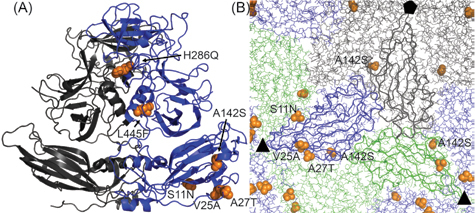
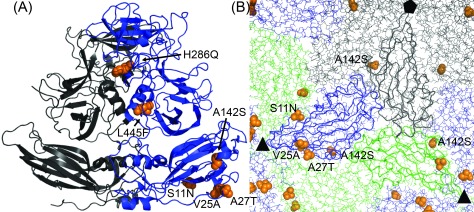
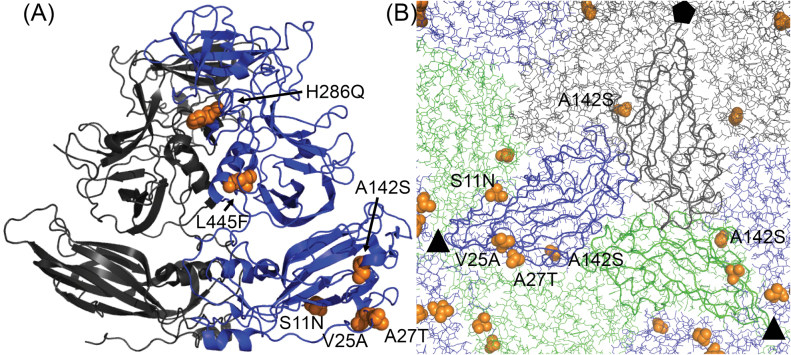
Norovirus GI.1 Norwalk and GI.1 West Chester capsid protein VP1 show six dissimilar substitutions. (A) Cartoon structure of a Norwalk VP1 dimer (PDB: 1IHM, residues 10–520) with quasi-equivalent monomers A (grey) and B (blue). Amino acids substituted for similar residues are not shown. Only the B subunit has a sufficiently resolved N-terminal arm to observe all four dissimilar amino acids in the S domain (bottom). The upper P domain only contains two major substitutions at the intra-dimer interface. All dissimilar residues are depicted as orange spheres and the substitution from Norwalk to West Chester is annotated. (B) S domains in the capsid context viewed from the interior with one subunit each (A—grey, B—blue, C—green) highlighted as ribbon. All substituted residues locate to the inter-dimer interface. Threefold and fivefold symmetry axes are labelled with black symbols.