Figure 3.
Control of Translation Initiation by eIF2 and Host Regulatory Kinases that Phosphorylate Its α subunit
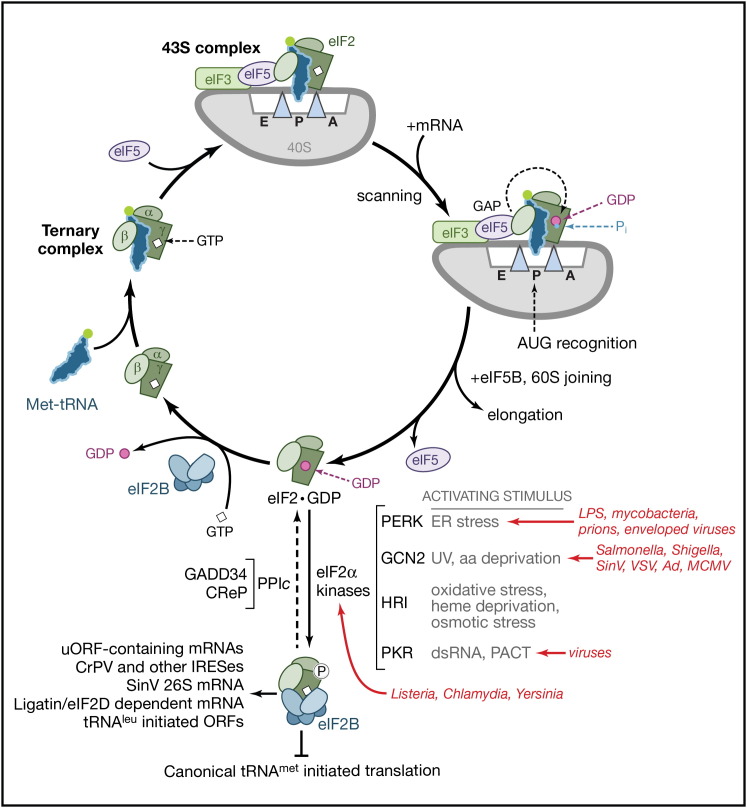
A ternary complex, comprised of eIF2 (α, β, and γ subunits, depicted) and GTP bound to the initiator-methionine tRNA (Met-tRNAi), loads Met-tRNAi into the ribosomal P site and forms a 43S preinitiation complex. After recruitment of eIF4F- and poly(A)-binding protein (PABP)-bound mRNA and recognition of the AUG start codon by scanning ribosomes, the GTPase-activating protein eIF5 stimulates GTP hydrolysis, and 60S subunit joining triggers the release of eIF2·GDP and inorganic phosphate (Pi). The resulting 80S ribosome carries out the elongation phase (Figure 1B). Inactive, GDP-bound eIF2 (eIF2·GDP) is recycled to the active GTP-bound form by the five-subunit guanine nucleotide exchange factor eIF2B.
Four different cellular eIF2α kinases (described in the main text), each of which is activated by a discrete stress, phosphorylate eIF2α, and prevent eIF2 recycling. Phosphorylated eIF2 binds tightly to and inhibits eIF2B, blocking canonical tRNAmet-initiated translation. When bound to either the inducible (growth arrest and DNA damage-inducible protein 34 [GADD34]) or constitutively active (CReP) regulatory subunit, the host protein phosphatase 1 catalytic (PP1c) subunit dephosphorylates eIF2. Pathogens shown in red and the different eIF2α kinases they activate are indicated. Viral (CrPV and other IRESs, Sindbis 26S mRNA) and host (uORF-containing, eIF2D-dependent, tRNAleu-initiated) mRNAs that are translated in the presence of phosphorylated eIF2 are indicated. SinV, Sindbis virus; VSV, vesicular stomatitis virus; Ad, adenovirus; CrPV, cricket paralysis virus; uORF, upstream open reading frame; aa, amino acid.