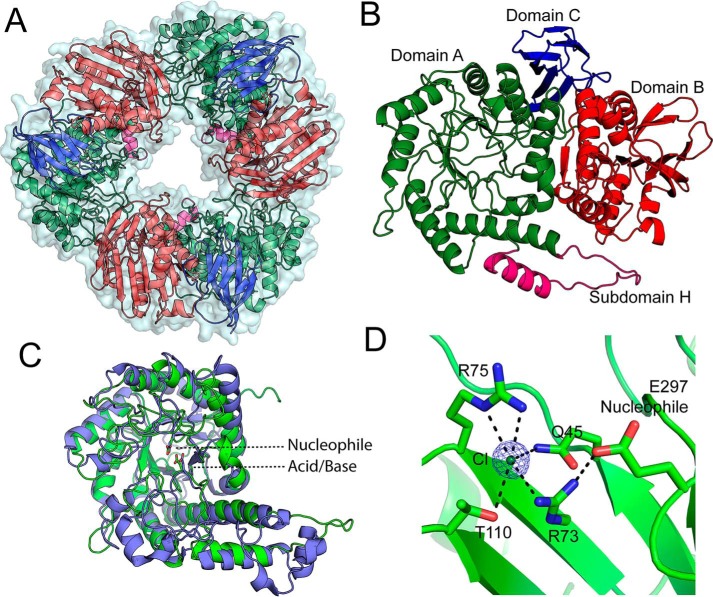
Figure 4.
Structure of Bs164. The trimeric structure of Bs164 is shown in panel A. All three protomers are shown with a surface and each chain is displayed as a cartoon diagram colored by domain. Panel B shows the structure of one protomer. Domain A, which has a (β/α)8-fold, is shown in green with subdomain H shown in magenta, domain B, containing a mixed β-sheet, is shown in red, and the β-sandwich of domain C is shown in blue. Overlay of domain A of Bs164 and the catalytic domain of β-galactosidase from Rahnella sp. R3 (5E9A) is shown in panel C. Bs164 is in green, whereas 5E9A is colored blue. Both the catalytic nucleophile and acid/base residue of both structures are shown as sticks. The chlorine-binding site of Bs164 is shown in D, with dashed lines showing polar interactions closer than 3.4 Å apart. Electron density for the chloride is a σA-weighted 2Fo − Fc density contoured at 3 σ and rendered with the program PyMol. The nucleophile residue Glu-297 is also shown as it interacts with Arg-73.