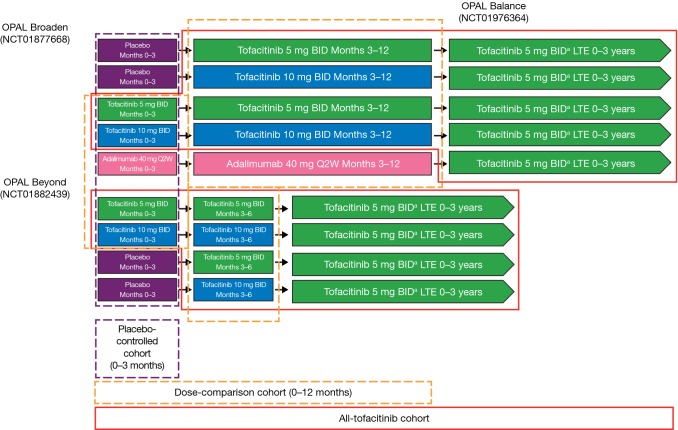
Fig. 1.
Schematic of clinical trial cohorts. aAll patients received tofacitinib 5 mg BID upon entry into OPAL Balance; the tofacitinib dose could be increased to 10 mg BID at the investigator’s discretion if it was believed that a patient would benefit from a higher dose and was not experiencing any tofacitinib-related AEs, including abnormalities in laboratory test results that were judged to be related to tofacitinib. The dose could be decreased from 10 to 5 mg BID for safety reasons at any time. AEs adverse events, BID twice daily, LTE long-term extension, Q2W every 2 weeks